Водород при Химиотерапия на Белодробен КарциномНаучно Изследване
Терапията с водороден газ подобрява преживяемостта на пациенти с рак на белия дроб
В наскоро проведено проучване терапията с водороден газ е помогнала на пациенти с рак на белия дроб да живеят по-дълго, като е направила имунните им клетки по-ефективни.
Проучването включва 56 пациенти с рак на белия дроб, които са лекувани с медикамента ниволумаб. Това е лекарство, което помага на имунната система да се бори с рака. От общия брой пациенти 42-ма са вдишвали и водороден газ в продължение на три часа всеки ден.
Всички пациенти са предоставили писмено информирано съгласие преди да участват в проучването. Неговият протокол е одобрен от комисиите за институционален контрол в Регионалния медицински център Тамана, Япония.
Изследователите разделят пациентите на две групи. Едната получава само ниволумаб. Другата група пациенти получава инхалации с водород и ниволумаб. От пациентите са взети кръвни проби, за да се изследва въздействието върху специфични имунни клетки, и са измерени нивата на CoQ10 – вещество, важно за клетъчната енергия.
Кои са двата вида клетки, важни при това проучване, и какъв е ефектът от водородната терапия?
Здрави имунни клетки
Те атакуват и унищожават раковите клетки. С течение на времето могат да се изтощят и да станат по-малко ефективни. Наричат се CD8+ Т клетки.
Изтощени имунни клетки
Те са загубили голяма част от способността си да се борят с рака. Наричат се PD-1+ Tim-3+ клетки.
CoQ10 е молекула, която помага на клетките да произвеждат енергия.
По-високите нива на CoQ10 показват по-добра клетъчна функция.
Какво прави водородната терапия?
Терапията с водород помага за намаляване на броя на изтощените клетки и увеличава производството на енергия в здравите имунни клетки, което ги прави по-ефективни в борбата с рака.
Резултати от проучването с водородна терапия при рак на белия дроб
Резултатите са обещаващи. Пациентите, които вдишват водород, живеят значително по-дълго от тези, които не го правят. Средната продължителност на преживяемостта за групата, получаваща и водородни инхалации, и ниволумаб, е 28 месеца, в сравнение с едва 9 месеца за групата, която получава само ниволумаб. Това показва, че комбинираната терапия е била около три пъти по-ефективна за удължаване на живота на пациентите.
Освен това проучването показва, че водородният газ сам по себе си също може да подобри резултатите за пациентите, което подчертава потенциала му като мощно средство за борба с рак. Терапията е помогнала за подмладяване на изтощените имунни клетки. Това откритие предполага, че водородният газ сам по себе си има благоприятни ефекти, които биха могли да допълнят конвенционалните терапии за лечение на рак.
Заключение
Добавянето на терапия с водород към лечението с ниволумаб може значително да удължи живота на пациентите с рак на белия дроб чрез повишаване на енергията и ефективността на техните имунни клетки.
Тази обещаваща терапия може да подобри резултатите за много пациенти, а ползите от водорода са очевидни дори когато се използва самостоятелно, което подчертава потенциала му като ценно допълнение към протоколите за лечение на рак.
Пълно съдържание на доклада:
оригинално заглавие (букв. прев.): Водородният газ активира коензим Q10 за възстановяване на изтощените CD8 + Т клетки, особено PD-1 + Tim3 + терминални CD8 + Т клетки, което води до по-добри резултати от ниволумаб при пациенти с рак на белия дроб
Junji Akagi, Hideo Baba
Department of Surgery, Tamana Regional Health Medical Center, Kumamoto 865‑0005, Japan, Department of Gastroenterological Surgery Kumamoto University, Kumamoto 860‑8556, Japan
-
Резюме:
Както беше съобщено по-рано, водородният газ подобрява прогнозата на пациенти с рак, като възстановява изтощените CD8+ Т клетки в активни CD8+ Т клетки , вероятно чрез активиране на митохондриите. Тъй като митохондриалните активатори проявяват синергични ефекти с ниволумаб, настоящото проучване изследва дали водородният газ също влияе върху клиничните резултати от ниволумаб. Общо 42 от 56 пациенти с рак на белия дроб, лекувани с ниволумаб, са получили водороден газ. Изчерпаните маркери (PD-1 и Tim-3) върху клетъчни популации в пътя на CD8+ Т клетъчна диференциация бяха анализирани с помощта на поточна цитометрия. Концентрацията на коензим Q10 (CoQ10) е измерена като маркер за функцията на митохондриите. 42-те пациенти, лекувани с водороден газ и ниволумаб (HGN), показват значително по-дълга обща преживяемост (OS) в сравнение с тези, лекувани само с ниволумаб (n=14). При многовариантен анализ, PD-1+Tim-3+терминални CD8+ Т клетки (PDT+) са независим лош прогностичен фактор за OS и CoQ10 показва тенденция да се свързва с подобрена OS. Промяната в степента на PDT+ и CoQ10 след спрямо преди HGN (съотношение на PDT+ и съотношение CoQ10, съответно) разкрива, че пациентите с ниско съотношение на PDT+ (<0,81) и високо съотношение на CoQ10 (>1,175) имат значително по-дълга OS в сравнение с тези с високо съотношение PDT+ и ниско съотношение на CoQ10. Освен това PDT+, със значителна обратна корелация с CoQ10, е значително по-нисък при пациенти с високо съотношение на CoQ10 и/или CoQ10, отколкото при тези с нисък CoQ10 и/или CoQ10. Предполага се, че водородният газ повишава клиничната ефикасност на ниволумаб чрез увеличаване на CoQ10 (митохондриите) за намаляване на PDT+, като PDT+ и CoQ10 са съответно надеждни отрицателни и положителни биомаркери на nivolumab.
Оригинална ПубликацияAbstract
As previously reported, hydrogen gas improves the prognosis of patients with cancer by restoring exhausted CD8+ T cells into active CD8+ T cells, possibly by activating mitochondria. As mitochondrial activators exhibit synergistic effects with nivolumab, the current study investigated whether hydrogen gas also affects the clinical outcomes of nivolumab. A total of 42 of 56 patients with lung cancer treated with nivolumab received hydrogen gas. Exhausted markers (PD‑1 and Tim‑3) on cell populations in the CD8+ T cell differentiation pathway were analyzed using flow cytometry. The concentration of coenzyme Q10 (CoQ10) was measured as a marker of mitochondrial function. The 42 patients treated with hydrogen gas and nivolumab (HGN) indicated a significantly longer overall survival (OS) compared with those treated with nivolumab only (n=14). In multivariate analysis, PD‑1+Tim‑3+terminal CD8+ T cells (PDT+) were an independent poor prognostic factor in OS, and CoQ10 showed a tendency to be associated with improved OS. The change in the rate of PDT+ and CoQ10 after vs. before HGN (PDT+ ratio and CoQ10 ratio, respectively) revealed that patients with low PDT+ ratio (<0.81) and high CoQ10 ratio (>1.175) had significantly longer OS compared with those with high PDT+ ratio and low CoQ10 ratio. Furthermore, PDT+, with a significant reverse correlation with CoQ10, was significantly lower in patients with high CoQ10 and/or CoQ10 ratio than in those low CoQ10 and/or CoQ10. Hydrogen gas has been suggested to enhance the clinical efficacy of nivolumab by increasing CoQ10 (mitochondria) to reduce PDT+, with PDT+ and CoQ10 as reliable negative and positive biomarkers of nivolumab, respectively.Introduction
Tumors expressing PD-L1 bind to PD-1, an immunoinhibitory receptor expressed on T cells, and inhibit T cell-mediated immune responses (1). Nivolumab (anti-PD-1 antibody) binds to PD-1 and blocks its PD-1/PDL-1 interaction. Nivolumab has led to extremely significant results in patients with terminally-progressed carcinomas who can be administered several treatments. However, because nivolumab is reported to have a low cure rate of 20–30%, many researchers have been searching for biomarkers that can be used to distinguish responders from non-responders. T cell exhaustion, a state of T cell dysfunction characterized by reduced cytokine production, impaired killing, and hypo-proliferation, was first characterized in chronic lymphocytic choriomeningitis virus (LCMV) infection (2,3). These T cell dysfunctions of exhausted T cells are inversely correlated with decreased mitochondrial function (3), which is caused by progressive loss of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), a regulator of mitochondrial replication that is controlled by a variety of signaling pathways (Akt, p38-MARK, AMPK, SIRT1, PRMT1) (4), likely driven by chronic antigen exposure in the tumor microenvironment (5). Honjo et al, reported that mitochondrial activation chemicals such as Bezafibrate, an activator of PGC1-α, synergize with nivolumab for T cell-dependent antitumor activity (6), suggesting that T cells with severe metabolic insufficiency equivalent to exhausted T cells are highly involved in low clinical outcomes of nivolumab and reinvigoration of exhausted T cells is incompletely achieved by checkpoint blockade such as nivolumab only, and consequently, metabolic reprogramming must be preceded for enhancement of checkpoint blockade (5). Recently, we reported that the proportion of PD-1+terminal CD8+ T cells containing PDT+ and PDT- (exhausted CD8+ T cells) in the peripheral blood of colorectal cancer patients was reduced by hydrogen gas, an activator of PGC1-α, leading to better prognosis (7). Furthermore, enforced expression of PGC1α in exhausted T cells results in superior antitumor T cell function (5,8). These reports indicate that mitochondrial activation chemicals, especially an activator of PGC1-α, such as hydrogen gas and Bezafibrate, enhance nivolumab outcomes by restoring exhausted T cells.
T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) is a type I membrane glycoprotein. Its expression can be found on terminally differentiated Th1 cells and innate immune cells (9–11). Recently, Tim-3-expressing PD-1+ terminal CD8+ T cells (PDT+) were reported to have the most severe exhaustive phenotype and most suppressive function compared with PD-1+ Tim-3−terminal CD8+ T cells (PDT−) (12), suggesting that mitochondrial dysfunction of PDT+ is more severe than that of PDT− and thus may be more responsible for nivolumab’s clinical effects than exhausted T cells expressing only PD-1. Some of Tim-3-expressing T cells have been recognized as senescent T cells characteristic of their irreversible dysfunction, and so it is doubtful whether hydrogen gas can restore PDT+ into active CD8+ T cells like the PD-1+terminal CD8+ T cells as described before. Restoring exhausted T cells, including PDT+, represents an inspiring strategy in cancer treatment with nivolumab and has yielded promising results. In the patients with advanced carcinomas, exhausted T cells are increased, resulting in low effectiveness of nivolumab (about 20–30% response rate), because of their dysfunctional cytotoxic activity. Hydrogen gas can restore the exhausted T cells into active T cells, which will bring a higher clinical response rate of nivolumab in the advanced cancer patients. Therefore, such restoration of exhausted CD8+ T cells by hydrogen gas has become a significant breakthrough in cancer immunotherapy with nivolumab.
Molecular hydrogen (i.e., dihydrogen or H2) was previously reported to efficiently neutralize hydroxyl radicals (•OH), but not other reactive oxygen species, such as superoxide anions (O2•-), hydrogen peroxide (H2O2), and nitric oxide (NO•) (13). As hydrogen gas is reported to activate PGC1-α (14), it is also one of the mitochondrial activation mediators. Recently, we reported that hydrogen gas could restore exhausted CD8+ T cells to active CD8+ T cells via mitochondrial activation (7). This implies that hydrogen gas may improve the clinical effects of nivolumab, as reported by Chamoto et al (6). However, there is no direct evidence that hydrogen gas activates mitochondria. Therefore, we have been searching for methods that clinically and easily measure mitochondrial function. Recently, coenzyme Q10 (CoQ10), a key enzyme of the mitochondrial respiratory chain, became easily and clinically measurable through the use of peripheral blood (15). CoQ10 transfers electrons from complexes I and II into complex III, which is a critical process for ATP production. CoQ10 supplementation was reported to activate mitochondrial function (16), which is reported to depend on the CoQ10 concentration in peripheral blood (17). Such findings suggest that the concentration of CoQ10 in peripheral blood is available as a marker of mitochondrial function. Therefore, we sought to use CoQ10 as a marker of mitochondrial function in this study.
Herein, we investigated whether hydrogen gas, an activator of PGC1-α, is able to enhance the clinical effect of nivolumab, and what its mechanism might be. Further, we investigated whether the CoQ10 concentration in the peripheral blood of cancer patients is available as a marker of mitochondrial function.
Materials and methods
Patients, sample collection and processing
All participants provided written informed consent prior to enrollment. The study protocol was approved by the Institutional Review Boards at the Tamana Regional Health Medical Center (Tamana, Kumamoto, Japan). All methods and procedures were consistent with Good Clinical Practice, the Declaration of Helsinki, and local laws. In this prospective cohort study, 56 patients with histologically- and clinically-diagnosed stage IV lung carcinoma, based on the unified Tumor-Node-Metastasis criteria (18), were enrolled at Tamana Regional Health Medical Center between July 2016 and July 2018. The specific exclusion and inclusion criteria were: A performance status of ≥2 and <2, hemoglobin of <8.0 and ≥8.0, WBC of <2,000 and ≥2,000, platelet of <60,000 and ≥60,000, respectively. Among the patients with lung carcinoma, 22 were men, and 34 were women, ranging in age from 33 to 84 years (mean age of 63.6±1.88 years). Patients were continuously treated with nivolumab (1 mg/kg) every 2 weeks. Patients also inhaled hydrogen gas 3 h daily at their home through a cannula or mask that they rented or purchased and connected to a Hycellvator ET 100 (Helix Japan, Co., Ltd.). None of the patients reported any complaints regarding the daily 3-h hydrogen gas inhalation. Peripheral blood (10 ml) was collected from patients prior to and every month after treatment with hydrogen gas.
When the total patients number reached to about 30, we found that the survival rate and QOL (Quality of life) of the patients treated with both nivolumab and hydrogen gas were quite better that those with nivolumab only. Therefore, then, we are sorry to select the combined treatment of nivolumab and hydrogen gas more preferably than the treatment of nivolumab only. That is why the number of the patients treated with combined treatment was more than those with nivolumab only. However, this result strongly suggest that the combined treatment of nivolumab and hydrogen gas is more clinically effective than the treatment with nivolumab only.
Hydrogen gas treatment
The Hycellvator ET 100 (Helix Japan, Co., Ltd.) generates 1.67 l/ min hydrogen gas (hydrogen purity, 99.99%) by electrolysis. As measured by gas chromatography at Kureha Special Laboratory, the gas generated 680,000 ppm hydrogen gas and 320,000 ppm oxygen gas. Recently, hydrogen gas inhalation was used in patients with post-cardiac arrest syndrome, and adverse events were not observed (19). Similarly, no adverse events were observed in the 56 patients who inhaled hydrogen gas for up to a maximum of 60 months in the present study.
Antibodies and fluorescence-activated cell sorting (FACS)
Briefly, Ficoll-Hypaque solution (20 ml) was placed into a 50-ml conical centrifuge tube using a sterile pipette. Anti-coagulated blood (10 ml) mixed with an equal volume of PBS was then slowly layered over the Ficoll-Hypaque solution by gently pipetting down the side of the tube. Samples were then centrifuged at 400 × g and 22°C for 30–40 min. Mononuclear cells that accumulated at the interface between the plasma (upper) and Ficoll-Hypaque layers (bottom) were carefully recovered using a Pasteur pipette and transferred to a 15-ml conical tube. Cells were then analyzed on a BD FACSCalibur (Nippon Becton Dickinson) with BD CellQuest software (v5.1) using anti-CD57 conjugated to fluorescein isothiocyanate (clone NK-1; cat. no. 347393; Nippon Becton Dickinson), mouse anti-human CD27 conjugated to APC (clone M-T271; cat. no. B09983; Beckman Coulter), mouse anti-human PD-1 conjugated to PE (clone EH12.1; cat. no. 557946; Nippon Beckton Dickinson), mouse anti-human Tim-3 conjugated to PE (clone FAB2365C; cat. no. 344823; R&D system), and mouse anti-human CD8 conjugated to PerCP (clone SK1; cat. no. 347314) (BD Pharmingen). The mixtures were incubated at 4 C for 30 min after blocking with 1% γ-globulin for 15 min at 4°C. To determine the independent contributions of each marker to PFS and OS, FACS data were used to stratify patients based on the proportion of early, intermediate, terminal, and end PD-1+ and PD-1− CD8+ T cells. All blood samples obtained from patients were transferred to SRL, Inc. for lymphocyte separation and flow cytometry; the status of the laboratory data, reliable protocols, and flow cytometry assays were certified by SRL, Inc., one of the most reliable clinical laboratory centers in Japan. The flow cytometry data were analyzed using SPSS v19.0 for Windows (IBM Corp.).
Measurement of Coenzyme Q10
Serum ubiquinol content was determined by LC/MS/MS (outsourced to Kaneka Techno Research Corporation). Briefly, 0.7 ml of isopropanol was added to 0.1 ml of serum. The obtained mixture was then centrifuged, and the resulting supernatant was filtered through a membrane filter. The obtained filtrate was then used as the sample for LC/MS/MS, which was performed on a Triple Quad5500 (AB SCIEX Company).
Study endpoints and assessments
Primary endpoints were PFS and OS time, and these were measured from the date of randomization to the first recurrence and mortality, regardless of cause, respectively. Patients were monitored by dynamic computed tomography or magnetic resonance imaging every 3 months from baseline up to 60 months, and every 3–6 months thereafter. Two independent and blinded radiologists, each with >5 years of experience, reviewed all scans at each site. When there was discord, the radiologists reviewed the images to reach the same conclusion following a discussion. Adverse events were classified and graded every 2 months according to the Common Terminology Criteria for Adverse Events v3.0 (National Cancer Institute) (20) from the day of consent until the end of the study, at least 30 days after treatment. Multiple events were counted once for each patient, of which the most severe was noted.
Statistical analysis
Significant differences between two groups were found using the (Mann-Whitney test). For persistent abnormal distribution, the linear correlation between two continuous variables was tested using Spearman’s correlation coefficient. Statistical analysis of Tables I–III was performed using χ2 test for comparing two groups. The receiver operating characteristic (ROC) analysis was used to determine the optimal cut-off values for continuous variables. The ROC curve shows 1-specificity on the x-axis and sensitivity on the y-axis. The optimal cut-off value was calculated by maximizing the sensitivity and specificity across various cut-off points on the ROC curve. The probability of survival was estimated by the Kaplan-Meier method, and differences in survival were evaluated by the log-rank test. Prognostic factors were tested by univariate and multivariate Cox regression analyses. All statistical analyses were performed using SPSS version 19.0 for Windows (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Table I.
Comparison of clinicopathological data between patients treated with Hydrogen gas + Nivolumab and Nivolumab only.
Table III.
Comparison of clinicopathological data between patients with high- and low-level of Coenzyme Q10.
Results
Hydrogen gas extends the OS of patients treated with hydrogen gas and nivolumab
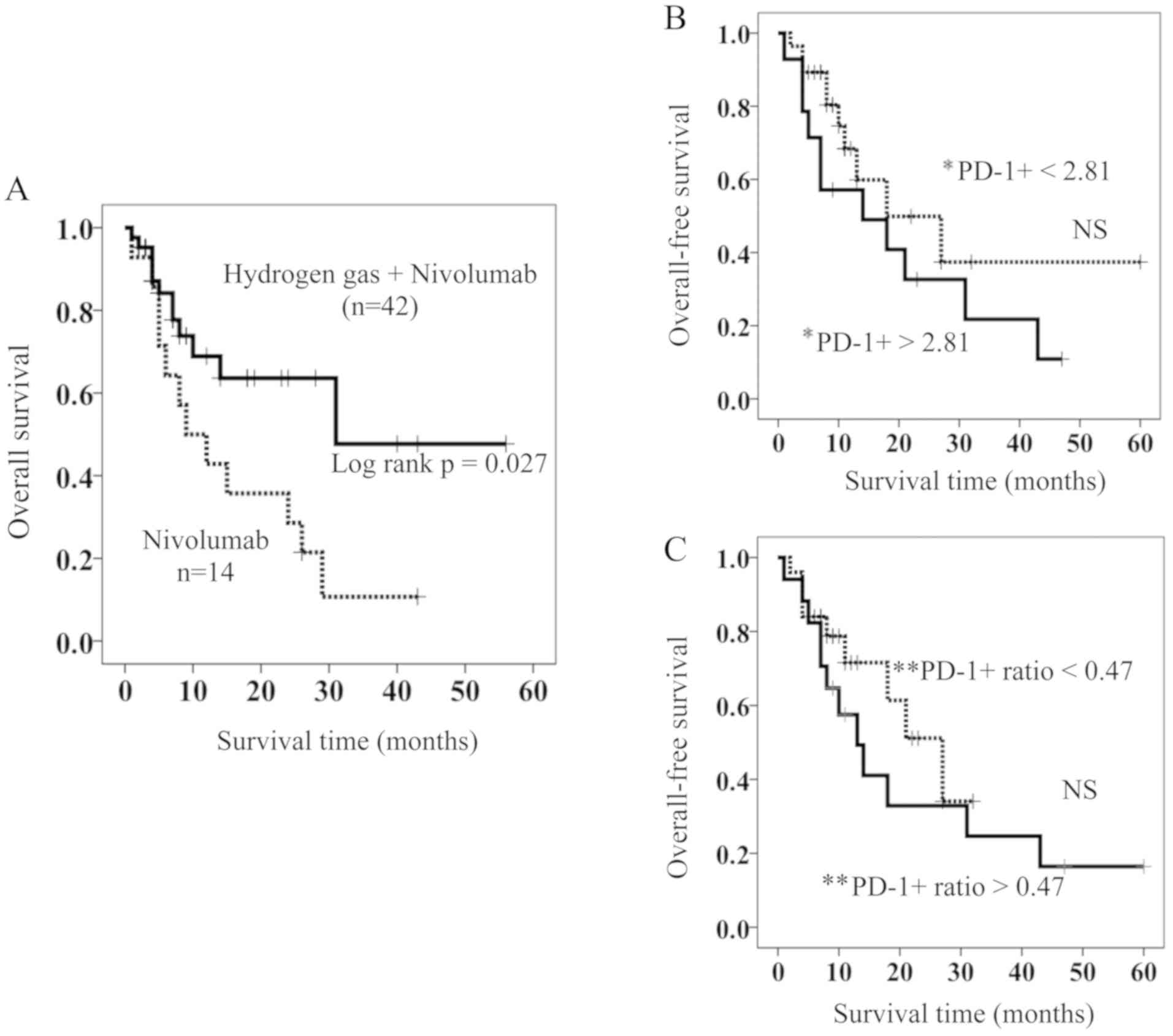
Of the patients with stage IV lung cancer, 42 were treated with hydrogen gas and nivolumab (HGN), while 14 were treated with nivolumab only (NO). Table I shows that a significant difference was not found in the clinico-pathological parameters such as age, gender, T (Tumor) factor (18), N (Node) factor (18), M (Metastasis) factor (18), and histology between the two groups. Kaplan-Meyer analysis showed that the HGN-treated patients had a significantly longer OS than those treated with NO (Fig. 1A). Median survival time (MST) for the HGN-treated patients was 28 months, a length that is approximately 3-fold longer than that for NO-treated patients (MST 9 months) (Fig. 1A).
Figure 1.
(A) OS in patients treated with hydrogen gas and nivolumab (solid line) and those treated with nivolumab only (dotted line). (B and C) Comparison of OS in HGN-treated patients with high (solid line) and low (dotted line) of (B) PD-1+ and (C) PD-1+ ratio. *PD-1+ =PD-1+terminal CD8+ T cells, **PD-1+ ratio =PD-1+terminal CD8+ T cells after treatment / PD-1+terminal CD8+ T cells before treatment. OS, overall survival; HGN, Hydrogen gas and Nivolumab; PD-1+, PD-1+terminal CD8+ T cells; PD-1+ ratio; PD-1+terminal CD8+ T cells after HGN treatment / PD-1+terminal CD8+ T cells before HGN treatment.
Prognostic factors of HGN-treated patients
It was recently reported that PD-1+terminal CD8+ T cells are an independent poor prognostic factor of colorectal cancer patients and hydrogen gas brought them a better prognosis by reducing PD-1+terminal CD8+ T cells probably and increasing PD-1−terminal CD8+ T cells. However, in the HGN-treated patients, the proportion of PD-1+terminal CD8+ T cells and the change in the rate of PD-1+terminal CD8+ T cells after vs. before HGN (PD-1+ ratio) were not involved in overall survival (Fig. 1B and C). As described in the introduction, PD-1+terminal CD8+ T cells (exhausted CD8+ T cells) are supposed to contain the most exhausted CD8+ T cells (PD-1+Tim-3+terminal CD8+ T cells, PDT+) as well as PD-1+Tim-3−terminal CD8+ T cells (PDT-), and so we sought to determine whether the expression of Tim-3 and/or PD-1 on populations of CD8+ T cells in differentiation pathways is associated with their OS.
Cox proportional-hazards regression analysis was used to identify PD-1+/− Tim-3+/− CD8+ T cell subsets and the clinico-pathological factors (age, sex, primary tumor (T), regional lymph nodes (N), distant metastasis (M), and histology) associated with OS in patients with stage IV lung cancer. By performing a univariate analysis of 17 factors, PD-1+Tim-3−intermediate CD8+ T cells (HR, 1.035; 95% CI, 1.008–1.062; P=0.009), PD-1+Tim-3+intermediate CD8+ T cells (HR, 2.504; 95% CI, 1.472–4.262; P=0.001), PD-1+terminal CD8+ T cells (HR, 1.092; 95% CI, 1.005–1.188; P=0.038), PD-1+Tim-3−terminal CD8+ T cells (HR, 1.089; 95% CI, 1.013–1.170; P=0.021), and PDT+ (HR, 49.97; 95% CI, 6.369–392.1; P<0.0001) was found to be significantly associated with poorer OS, while PD-1 Tim-3−intermediate CD8+ T cells (HR, 0.966; 95% CI, 0.942–0.991; P=0.008), PD-1− Tim-3−terminal CD8+ T cells (HR, 0.922; 95% CI, 0.8680.980; P=0.009), and CoQ10 (HR, 0.717; 95% CI, 0.541–0.949; P=0.020) with better OS. On the other hand, four types of PD-1−Tim-3+early-, intermediate-, terminal-, and end-diff. CD8+ T cells were not significantly associated with OS. Based on multivariate Cox regression, terminal PDT+ was more strongly associated with OS than others (HR, 27.16; 95% CI, 3.152–233.9; P=0.003), and CoQ10 showed a tendency to be associated with better prognosis (HR, 0.780; 95% CI, 0.602–1.012; P=0.062).
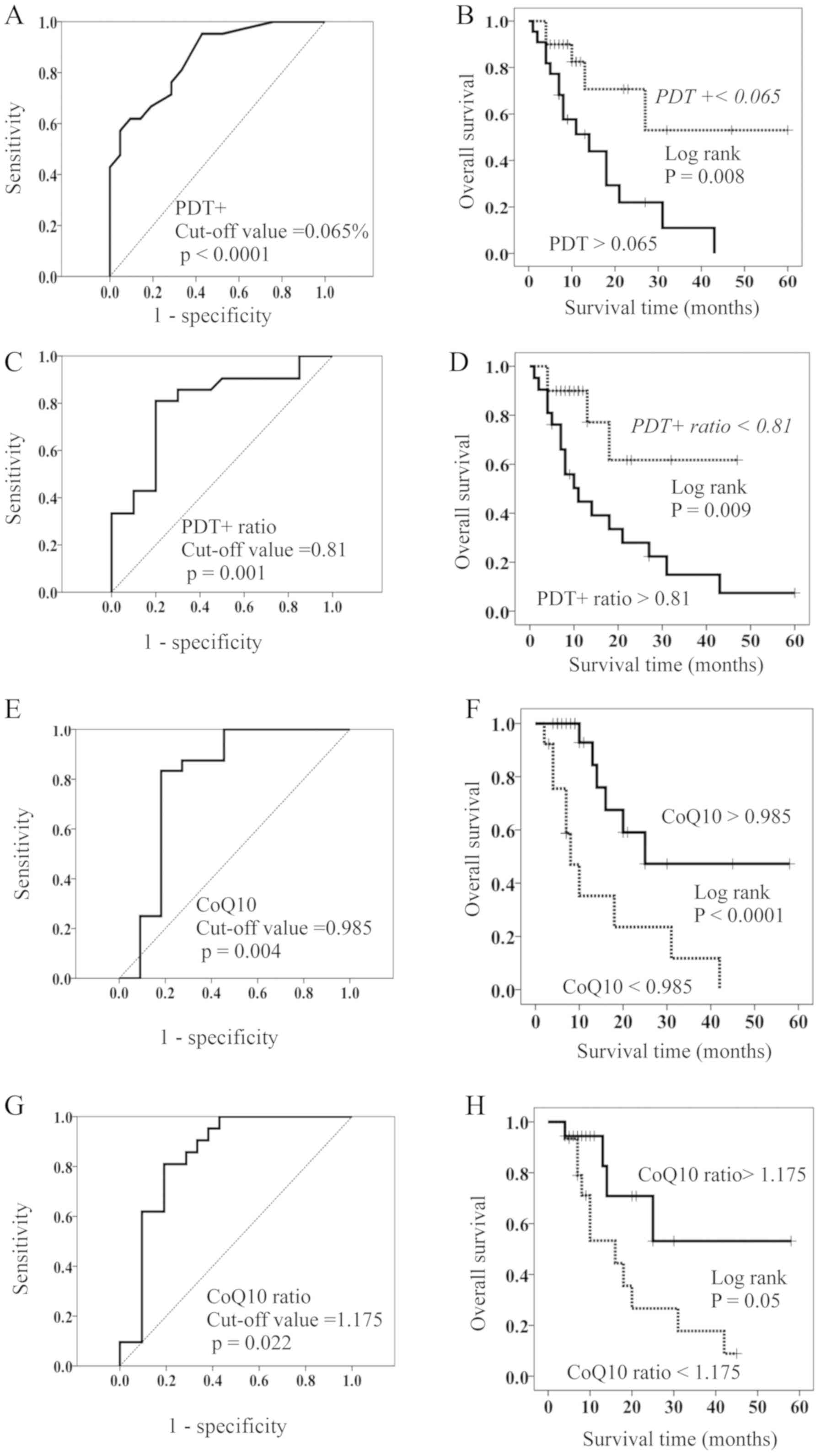
Based on the above results, patients were stratified as having high and low PDT+ by using a cut-off value of 0.065% for OS (ROC (Receiver Operating Characteristic) curve; AUC (Area Under the curve)=0.858, Sensitivity=76.2%, Specificity=71.4%) (Fig. 2A). There were no significant differences in the clinico-pathological factors between patients with high and low PDT+ (Table II); the resulting stratified OS curves are plotted in Fig. 2B. The curves demonstrated that patients with low PDT+ had a significant increase in OS compared to those with high PDT+. MST of OS was 11 months for patients with high PDT+ and 60 months for those with low PDT+ (Fig. 2B).
Figure 2.
(A) ROC of OS based on terminal PDT+. The cutoff value was 0.065%. (B) OS in patients with a high- (solid line) and low- (dotted line) proportion of PDT+. (C) ROC curves of OS based on the PDT+ ratio. The cutoff value was 0.81. (D) OS in patients with a high- (solid line) and low- (dotted line) PDT ratio. (E) ROC curves of OS based on the concentration of coenzyme Q10 (CoQ10). The cutoff value was 0.985 µg/ml. (F) OS in patients with a high- (solid line) and low- (dotted line) concentration of CoQ10. (G) ROC curves of OS based on the CQ10 ratio. The cutoff value was 1.175. (H) OS in patients with a high- (solid line) and low- (dotted line) CoQ10 ratio. OS, overall survival; PDT+, PD-1+Tim-3+ terminal CD8+ T cells; PDT+ ratio, PD-1+Tim-3+terminal CD8+ T cells after HGN treatment /PD-1+Tim-3+terminal CD8+ T cells before HGN treatment; CoQ10 ratio, CoQ10 after HGN treatment/CoQ10 before HGN treatment; ROC, Receiver operating characteristic curves.
Table II.
Comparison of clinicopathological data between patients with high- and low-level of PD-1+Tim-3+terminal CD8+ T cells (PDT+).
Hydrogen gas reduces PDT+, leading to better prognosis
Hydrogen gas decreased the proportion of PDT+ in 20 of the 42 patients (47.6%) and PD-1+Tim3−terminal CD8+ T cells (PDT-) in 23 of the 42 patients (54.8%). In contrast, it increased the proportion of PD-1−Tim3−terminal CD8+ T cells in 30 of the 42 patients (71.4%). We calculated the change in ratio for the proportion of PDT+ after HG vs. before HG (PDT+ ratio). The cut-off value of the PDT+ ratio was 0.81 according to ROC curve (AUC=0.796, Sensitivity=81.0%, Specificity=80.0%) (Fig. 2C), and patients with low PDT+ ratio showed a significantly longer OS than those with a high PDT+ ratio (Fig. 2D). The MST of the latter patients was 10 months, while more than 50% of the former are still alive (Fig. 2D).
Concentration of CoQ10 in the peripheral blood is highly associated with OS
PDT+ is assumed to have a more progressive exhaustion than PDT-, leading to a worse prognosis compared to PDT-. As the two exhausted CD8+ T cell populations (PDT+ and PDT-) are naturally considered to have mitochondrial dysfunction, with potential differences in their degree, measurement of mitochondria function is very important. While we are yet to derive a method that clinically and easily grasps mitochondrial function, the CoQ10 concentration can already be measured using peripheral blood. Because CoQ10 plays an important role in the mitochondrial respiratory chain, its concentration in peripheral blood may be an approximate value of mitochondrial function. We measured the CoQ10 concentration in the 35 HGN-treated patients with stage IV lung cancer and investigated its association with OS. The cut-off value was found to be 0.985 µg/ml (ROC curve; AUC=0.803, Sensitivity=83.3%, Specificity=81.8%, (Fig. 2E). Further, the 35 HGN-treated patients were stratified as having high (>0.985 µg/ml) and low (<0.985 µg/ml) CoQ10 concentrations. There were no significant differences in the clinico-pathological factors between patients with high and low CoQ10 (Table III). Patients with high CoQ10 had a significantly longer OS than those with low CoQ10 (Fig. 2F). MST of patients with high CoQ10 was 25 months, while that of patients with low CoQ10 was 8 months (Fig. 2F).
Following hydrogen gas treatment, the concentration of CoQ10 was increased in 19 (46%) of 41 patients (Table IV). Hence, we proceeded to calculate the rate of change of CoQ10 by hydrogen gas treatment (CoQ10 ratio=CoQ10 concentration after treatment/CoQ10 concentration before treatment) and classified patients by high- and low-CoQ10 ratio using the cut-off value (1.175) (ROC curve; AUC=0.710, Sensitivity=75.0%, Specificity=66.7%) (Fig. 2G). Patients with a high-CoQ10 ratio survived significantly longer than those with a low-CoQ10 ratio, as shown in Fig. 2H. MST of patients with a high CoQ10 ratio was NR (not reached to 50% overall survival), while that of patients with low CoQ10 was 9 months (Fig. 2H).
Table IV.
aPDT+ ratio and CoQ10 ratio.
Exhausted CD8+ T cells, especially PDT+, are highly associated with CoQ10
PDT+ might be more exhausted than PDT- and may have more severe mitochondrial dysfunction than PDT-. As described above, if the concentration of CoQ10 in the peripheral blood of patients can predict mitochondrial function to some extent, an association between the proportion of PDT+ and the concentration of CoQ10 may exist; this is because the most striking feature of PDT+ is mitochondrial dysfunction.
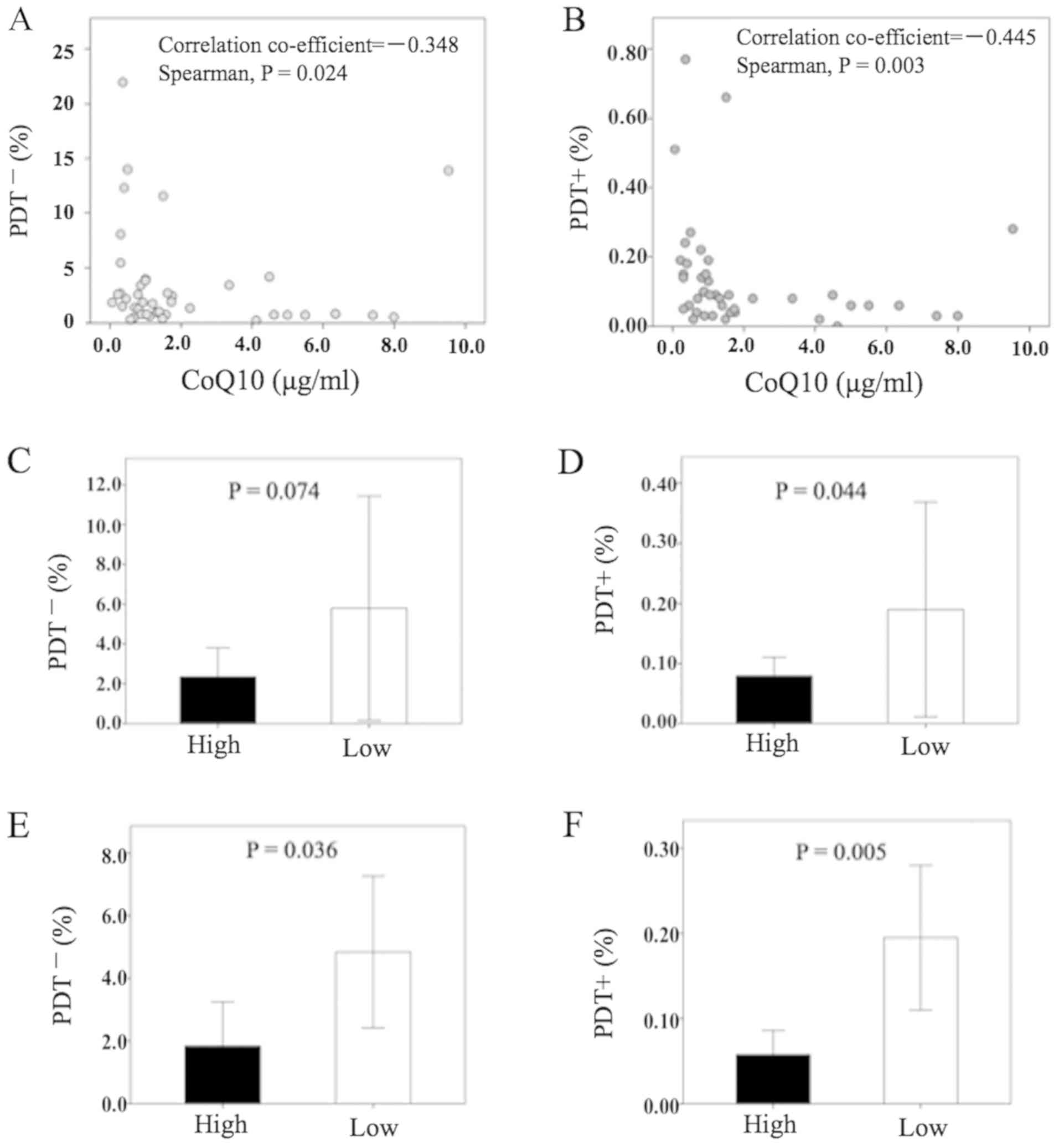
We found that PDT+ showed a moderate reverse correlation to CoQ10, which was more significant than that of PDT- (Fig. 3A and B). In patients with a high level of CoQ10, the proportion of PDT+ was significantly lower than in patients with low CoQ10 (Fig. 3D), while that of PDT- was not significant (Fig. 3C). Further, in patients with a high-CoQ10 ratio (i.e., patients with increased CoQ10 concentration after HGN-treatment), both of PDT- and PDT+ were significantly lower than in patients with a low-CoQ10 ratio (Fig. 3E and F, respectively). Table IV shows that the CoQ10 ratio was significantly low in patients with a high PDT+ ratio, while the CoQ10 ratio was significantly high in those with a low PDT+ ratio.
Figure 3.
(A) Point diagram of the correlation between CoQ10 concentration (x-axis) and PDT- (y-axis). (B) Point diagram of the correlation between CoQ10 concentration (x-axis) and PDT+ (y-axis). Comparison of (C) PDT- and (D) PDT+ between High and Low concentration of CoQ10. Comparison of (E) PDT- and (F) PDT+ between High and Low CoQ10 ratio. Mann-Whitney test was used to investigate statistical differences between two groups. PDT-, PD-1+Tim-3−terminal CD8+ T cells; PDT+, PD-1+Tim-3+ terminal CD8+ T cells; PDT+ ratio, PD-1+Tim-3+terminal CD8+ T cells after HGN treatment / PD-1+Tim-3+terminal CD8+ T cells before HGN treatment; CoQ10 ratio, CoQ10 after HGN treatment / CoQ10 before HGN treatment.
Discussion
In the present study, we revealed that hydrogen gas enhances the overall survival time of nivolumab-treated lung cancer patients. The MST of the patients treated with nivolumab and hydrogen gas (28 months) is about three times longer than that of those treated with nivolumab only (9 months). Although PD-1+terminal CD8+ T cells were an independent poor prognostic factor in the stage IV colorectal cancer patients as previously reported (7), the CD8+ T cells expressing both PD-1 and Tim-3 (PDT+) were most significantly responsible for OS of the patients treated with nivolumab and hydrogen gas, suggesting that nivolumab masks an expression of PD-1 molecules on CD8+ T cells and so measurement of Tim-3 molecules as well as PD-1 on CD8+ T cells is required for an accurate evaluation of prognosis of patients treated with nivolumab. Contrary to our recent report on PD-1+terminal CD8+ T cells (7), the hydrogen-induced reduction of the proportion of PD-1+terminal CD8+ T cells in this study did not cause an improvement of overall survival, but hydrogen gas decreased PDT+ and increased PD-1−Tim-3−terminal CD8+ T cells, which resulted in a better OS in HGN-treated patients. PDT+ is supposed to be the most severely exhausted CD8+ T cells possible exhibiting severe mitochondrial dysfunction, which is caused by progressive loss of PGC1α, a regulator of mitochondrial replication, which is controlled by a variety of signaling pathways (Akt, p38-MARK, AMPK, SIRT1, PRMT1) (4), likely driven by constitutive chronic antigen exposure in the tumor microenvironment (5). It was reported that hydrogen is associated with these signaling pathways (21–24), suggesting that it may improve PGC1-α through these pathways to enhance the clinical effects of nivolumab by restoring exhausted CD8+ T cells. Direct mitochondrial activators synergize with PD-1 blockade therapy; however, none of the mitochondrial activation chemicals alone exert any effects on tumor growth (6). This indicates that the better clinical effects observed in this study are not attributable to hydrogen gas alone, and as such, nivolumab is also required. This phenomenon may be explained by the fact that the reactive T cells by hydrogen gas contact cancer cells and subsequently express PD-1 in response to PDL-1 expressed on tumor cells, and consequently, nivolumab is required at the final stage of cancer attack. This is only the best situation for nivolumab showing its own best performance. Taken together, it is assumed that there are three kinds of PD-1-expressing CD8+ T cells, namely 1) CD8+ T cells transiently express PD-1 in response to PDL-1 on tumor cells and have normal mitochondria (referred to as suppressive CD8+ T cells, for convenience), 2) exhausted CD8+ T cells have mitochondrial disorder driven by a variety of signals from the cancer microenvironment and consequently express PD-1 (exhausted CD8+ T cells), and 3) more exhaustive CD8+ T cells express both PD-1 and Tim-3 and possess more severe mitochondrial disorder (PDT+). Suppressive CD8+ T cells are nivolumab-responder type, and both exhausted CD8+ T cells, especially PDT+, are nivolumab-non-responder type, requiring mitochondrial activation substances such as hydrogen gas for making nivolumab effective.
Because mitochondrial dysfunction of the two exhausted CD8+ T cells described above is caused by loss of PGC1-α and hydrogen gas activates PGC1-α, the hydrogen gas-induced reduction of the two exhausted CD8+ T cells (PDT- and PDT+) is assumed to be due to mitochondrial reactivation by hydrogen gas. However, there is currently no appropriate method to clinically and easily measure mitochondrial function. In this study, we attempted to use, as a marker of mitochondrial function, the concentration of CoQ10 in peripheral blood. This is because CoQ10 plays an important role in the mitochondrial respiratory chain, and it has recently become possible to easily measure its concentration in peripheral blood. In this study, we found that the CoQ10 concentration was highly associated with the better prognosis of lung cancer patients (Fig. 2F), which is the first report about an association of CoQ10 with the prognosis of cancer patients. Moreover, the hydrogen gas-induced increase in the CoQ10 concentration was found to result in an improvement in prognosis (Fig. 2H). The proportion of PDT+ was significantly lower in patients with a high CoQ10 concentration than in those with a low concentration (Fig. 3D). Additionally, when the CoQ10 concentration increased, that of PDT+ significantly decreased (Fig. 3F and Table IV). This finding suggests that the proportion of PDT+ is influenced by CoQ10 concentration. CoQ10 concentration in the peripheral blood of lung cancer patients was inversely associated with the proportion of PDT+, more significantly than with the proportion of PDT- (Fig. 3A and B). These results are indicative that the concentration of CoQ10 reflects mitochondrial function. Furthermore, exhausted CD8+ T cells, especially PDT+, may be an opposite marker of mitochondrial function. Consequently, it is suggested that hydrogen gas activates the mitochondria (CoQ10) to restore exhausted CD8+ T cells, including PDT+, into the active form of CD8+ T cells, resulting in better nivolumab outcomes. Our study revealed that nivolumab could not exert clinical effects against exhausted CD8+ T cells, especially PDT+, which is the major reason for the clinical response rate of nivolumab being as low as 20–30% in patients with advanced cancer. From this point of view, hydrogen gas has good compatibility with nivolumab as it decreases exhausted CD8+ T cells. Thus, PDT+ and CoQ10 might be reliable negative and positive biomarkers of nivolumab, respectively. Although coenzyme Q10 (CoQ10), a key enzyme of the mitochondrial respiratory chain (15), is suggested in this study to be a representative marker of mitochondrial function, we did not measure other mitochondrial enzymes such as nicotinamide adenine dinucleotide (NAD+), NADH, flavin adenine dinucleotide (FAD), acetyl coA and ATP amount in this study and so it is unclear yet whether a measurement of peripheral CoQ10 concentration is most suitable marker of mitochondrial function. Therefore, it will be required in the future to investigate whether CoQ10 is more suitable mitochondrial marker than other mitochondrial enzymes as described above. The present study had limitations. Other mitochondrial enzymes such as nicotinamide adenine dinucleotide (NAD+), NADH, flavin adenine dinucleotide (FAD), acetyl coA and ATP amount were not investigated in the present study and so it is unclear yet whether a measurement of peripheral CoQ10 concentration is most suitable marker of mitochondrial function.
Although pulmonary function tests before and after hydrogen is an important subject to evaluate patients’ prognosis, we did not performed pulmonary function tests and will investigate it in the future.
Hydrogen gas could not restore PDT+ in all of the patients. PDT+ was not reduced in 21 of 41 patients (51.0%) by hydrogen gas, and in 22 out of 41 patients (54%), hydrogen gas could not increase the CoQ10 concentration (Table IV). In approximately half of the lung cancer patients, hydrogen gas could not activate CoQ10 (mitochondria) and/or reduce PDT+. The existence of senescent CD8+ T cells, which are known to show decreased proliferation, defective mitochondrial function, and Tim-3 expression, has been reported (25). In addition, it was recently understood that senescent CD8+ T cells are not only dysfunctional CD8+ T cells, but they have an important role in altering the tissue microenvironment and affecting neighboring cells via the production and secretion of pro-inflammatory cytokines, chemokines, growth factors, and proteases through paracrine signaling (26). The most striking feature of senescent CD8+ T cells is that not only their T cell-dysfunction but also their mitochondrial dysfunction should be irreversible as reported before (25,27–29). In this study, there also existed hydrogen-unresponsive PDT+, which may be senescent CD8+ T cells. Nonetheless, recent studies have shown that even senescent T cells could regain function by inhibiting the p38 mitogen-activated protein kinase (MAPK) pathway (30,31), which is one of the MAPK subfamilies that regulate cell growth, differentiation, and stress responses (32). Further, it was reported that hydrogen gas inhibits the p-38 pathway (33,34), suggesting that hydrogen gas may even restore senescent CD8+ T cells (including hydrogen-unresponsive PDT+) into active CD8+ T cells by inhibiting the p38 pathway. Our hydrogen gas-related data revealed that the longer patients inhaled hydrogen gas, the better their prognosis (data not shown). Such finding suggests that a longer inhalation of hydrogen gas may cause further effective recoveries of PDT+ even if they are senescent CD8+ T cells. For example, there are few cases with a 10-h inhalation per day that demonstrate much better clinical effects than those with the usual 3-h inhalation per day.
When the total patients number reached to about 30, we found that the survival rate and QOL (Quality of life) of the patients treated with both nivolumab and hydrogen gas were quite better that those with nivolumab only. Therefore, then, we are sorry to select the combined treatment of nivolumab and hydrogen gas more preferably than the treatment of nivolumab only. That is why the number of the patients treated with combined treatment was more than those with nivolumab only. However, this result strongly suggest that the combined treatment of nivolumab and hydrogen gas is more clinically effective than the treatment with nivolumab only.
In conclusion, hydrogen gas activates CoQ10 (mitochondria), thereby enhancing the outcomes of nivolumab via reducing exhausted CD8+ T cells, especially PDT+. Our findings also suggest that CoQ10 concentration in peripheral blood is an available marker of mitochondrial function, while PDT+ and CoQ10 are reliable biomarkers of nivolumab. It is assumed that nivolumab is not effective for patients with predominant exhausted CD8+ T cells in peripheral blood, which can be overcome by hydrogen gas.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors’ contributions
HB interpreted the patient blood data. JA performed the blood examination of the patients with lung cancer, analyzed their data and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The ethic review board of Tamana Regional Health Medical Center approved the current study.
Patient consent for publication
All patients were provided with informed consent for publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
PDT+ PD-1+Tim3+terminal CD8+ T cells
PDT- PD-1+Tim3−terminal CD8+ T cells
PD-1 programmed cell death 1
PGC-1α peroxisome proliferator-activated receptor γ coactivator 1α.
Zou W, Wolchok JD and Chen L: PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 8:328rv42016. View Article : Google Scholar : PubMed/NCBI | |
Zajac AJ, Blattman JN, Murali-Krishna K, Soudive DJ, Suresh M, Altman JD and Ahmed R: Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 188:2205–2213. 1998. View Article : Google Scholar : PubMed/NCBI | |
Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G and Ferrari C: PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 80:11398–11403. 2006. View Article : Google Scholar : PubMed/NCBI | |
Fernandez-Marcos PJ and Auwerx J: Regulation of PGC1-α, a nodal regulation of mitochondrial biogenesis. Am J Clin Nutr. 93:884S–890S. 2011. View Article : Google Scholar : PubMed/NCBI | |
Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL and Delgoffe GM: The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 45:374–388. 2016. View Article : Google Scholar : PubMed/NCBI | |
Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S and Honjo T: Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci USA. 114:E761–E770. 2017. View Article : Google Scholar : PubMed/NCBI | |
Akagi J and Baba H: Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep. 41:301–311. 2019.PubMed/NCBI | |
Lund AW: Re-energizing exhausted T cells? Sci Transl Med. 8:353ec1352016. View Article : Google Scholar | |
Wang F, He W, Zhou H, Yuan J, Wu K, Xu L and Chen ZK: The Tim-3 ligand galectin-9 negatively regulates CD8+ alloreactive T cell and prolongs survival of skin graft. Cell Immunol. 250:68–74. 2007. View Article : Google Scholar : PubMed/NCBI | |
Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, et al: Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 318:1141–1143. 2007. View Article : Google Scholar : PubMed/NCBI | |
Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al: Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 205:2763–2779. 2008. View Article : Google Scholar : PubMed/NCBI | |
Sakuishi K, Apethoh L, Sullivan JM, Biazar BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 207:2187–2194. 2011. View Article : Google Scholar | |
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S and Ohta S: Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar : PubMed/NCBI | |
Kamimura N, Ichimiya H, Luchi K and Ohta S: Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1α to enhance fatty acid metabolism. NPJ Aging Mech Dis. 2:160082016. View Article : Google Scholar : PubMed/NCBI | |
Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K and Kitahara M: Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 47:19–28. 2007. View Article : Google Scholar : PubMed/NCBI | |
Tian G, Sawashita J, Kubo H, Nishio SY, Hashimoto S, Suzuki N, Yoshimura H, Tsuruoka M, Wang Y, Liu Y, et al: Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid Redox Signal. 20:2606–2620. 2014. View Article : Google Scholar : PubMed/NCBI | |
Gyozdiakova A, Kucharska J, Sumbalova Z, Nemec M, Chladekova A, Vancova O, Rausová Z, Kubalová M, Kuzmiaková Z and Mojto V: Platelets mitochondrial function depends on CoQ10 concentration in winter, not in spring season. Gen Physiol Biophys. 38:325–334. 2019. View Article : Google Scholar : PubMed/NCBI | |
Sobin LH and Wittekind CH: UICC TNM Classification of Malignant Tumors. John Wiley and Sons; New York, NY: 1997 | |
Tamura T, Hayashida K, Sano M, Suzuki M, Shibusawa T, Yoshizawa J, Kobayashi Y, Suzuki T, Ohta S, Morisaki H, et al: Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome-First-in-Human Pilot Study. Circ J. 80:1870–1873. 2016. View Article : Google Scholar : PubMed/NCBI | |
National Cancer Institute: Cancer therapy evaluation program, common terminology criteria for adverse events, v3. Int J Clin Oncol. 9 (Supp III):1–82. 2004. | |
Wang M, Tang W and Zhu YZ: An update on AMPK in hydrogen sulfide pharmacology. Front Pharmacol. 8:8102017. View Article : Google Scholar : PubMed/NCBI | |
Chi Q, Wang D, Hu X and Li S and Li S: Hydrogen sulfide gas exposure induces necroptosis and promotes inflammation through the MAPK/NF-κB pathway in broiler spleen. Oxid Med Cell Longev. 2019:80618232019. View Article : Google Scholar : PubMed/NCBI | |
Li S, Fujino M, Ichimaru N, Kurokawa R, Hirano S, Mou L, Takahara S, Takahara T and Li XK: Molecular hydrogen protects against ischemia-reperfusion injury in a mouse fatty liver model via regulating HO-1 and Sirt1 expression. Sci Rep. 8:140192018. View Article : Google Scholar : PubMed/NCBI | |
Zhang B, Zhao Z, Meng X, Chen H, Fu G and Xie K: Hydrogen ameliorates oxidative stress via PI3K-Akt signaling pathway in UVB-induced HaCaT cells. Int J Mol Med. 41:3653–3661. 2018.PubMed/NCBI | |
Crespo J, Sun H, Welling TH, Tian Z and Zou W: T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 25:214–221. 2013. View Article : Google Scholar : PubMed/NCBI | |
Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN and Henson SM: Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. 17:e126752018. View Article : Google Scholar | |
Vallejo AN, Weyand CM and Goronzy ZJJ: T-cell senescence: A culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 10:119–124. 2004. View Article : Google Scholar : PubMed/NCBI | |
Chappert P and Schwartz RH: Induction of T cell anergy: Integration of environmental cues and infectious tolerance. Curr Opin Immunol. 22:552–559. 2010. View Article : Google Scholar : PubMed/NCBI | |
Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C and Speiser DE: The three main stumbling blocks for anticancer T cells. Trends Immunol. 33:364–372. 2012. View Article : Google Scholar : PubMed/NCBI | |
Lanna A, Henson SM, Escors D and Akbar AN: The kinase p38 activated by the metablic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol. 15:965–972. 2014. View Article : Google Scholar : PubMed/NCBI | |
Lanna A, Henson M and Akbar A: The regulation of T cell senescence and metabolism by p38 map kinase signaling. Innov Aging. 1 (Suppl 1):12542017. View Article : Google Scholar | |
Arthur JS and Ley SC: Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 13:679–692. 2013. View Article : Google Scholar : PubMed/NCBI | |
Zhou J, Yan P, Zhu XD and Yu KJ: Hydrogen mitigates acute lung injury through upregulation of M2 and downregulation of M1 macrophage phenotypes. Int J Clin Exp Med. 11:7927–7935. 2018. | |
Li D and Ai Y: Hydrogen saline suppresses neuronal cell apoptosis and inhibit the p38 mitogen-activated protein kinase-caspase-3 signaling pathway following cerebral ischemia-reperfusion injury. Mol Med Rep. 16:5321–5325. 2017. View Article : Google Scholar : PubMed/NCBI |