H2 regulates miR-9, miR-21, and miR-199 in activated retinal microgliaScientific Research

Molecular hydrogen regulates the expression of miR-9, miR-21 and miR-199 in LPS-activated retinal microglia cells
Guo-Dan Liu
1Department of Ophthalmology, the Fourth Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
Hong Zhang
2Department of Ophthalmology, the First Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
Lin Wang
2Department of Ophthalmology, the First Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
Qing Han
1Department of Ophthalmology, the Fourth Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
Shi-Feng Zhou
2Department of Ophthalmology, the First Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
Ping Liu
2Department of Ophthalmology, the First Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
Abstract
AIM
To explore the potential mechanism of molecular hydrogen in the regulation of miRNA expression and signal-modulating activities.
METHODS
Retinal microglia cells were activated by Lipopolysaccharides (LPS) and then treated with hydrogen-saturated medium or normal medium without hydrogen. qRT-PCR was used to detect the expression difference in miR-9, miR-21 and miR-199 between these two groups. Moreover, the expression of LPS-induced signaling proteins, including Myd88, IKK-β, NF-κB, and PDCD4, were detected by Western blotting.
RESULTS
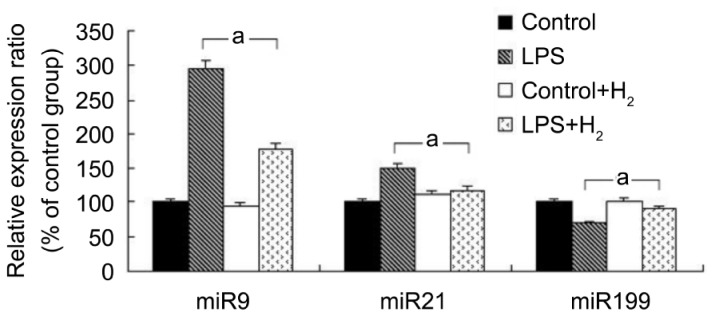
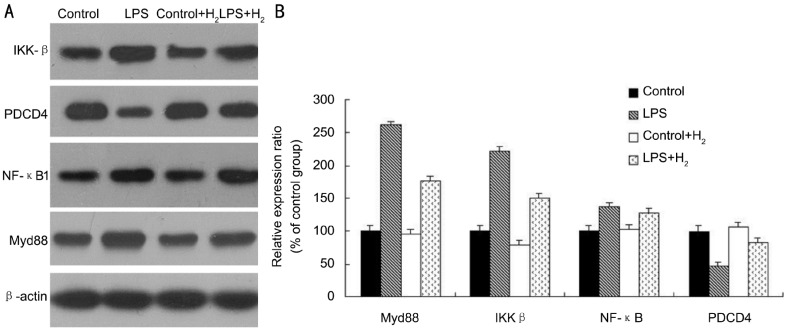
The results demonstrated a marked down-regulation of miR-9 and miR-21 and up-regulation of miR-199 by hydrogen treatment; the expression of Myd88 and IKK-β was decreased after hydrogen treatment, whereas PDCD4 was increased, and there was no significant change in NF-κB expression.
CONCLUSION
The results in the present study indicate that miR-9, miR-199 and miR-21 play an important role in the anti-inflammatory regulation of LPS-activated microglia cells by molecular hydrogen, which will help to explain the protective mechanism of molecular hydrogen against inflammatory injury.
INTRODUCTION
Diabetic retinopathy is a common complication of diabetes mellitus and a leading cause of adult blindness, which is characterized by the breakdown of the blood-retinal barrier, the loss of pericytes, the development of microaneurysms, exudative retinopathy and proliferative neovascularization. In modern medicine, the treatment of diabetic retinopathy is primarily focused on the hormones, cytokines and antioxidants that were proved to decrease retinal injury[1]–[3]. However, existing medicines are injected into either the eyes or the peritoneal cavity and are difficult to be delivered into the vitreous body, so there is no significant curative effect for diabetic retinal injury. Thus, easily applicable antioxidative reagents without significant side effects are strongly desirable. Previous studies demonstrated that molecular hydrogen (H2) was an efficient antioxidant gas that selectively reduced hydroxyl radicals (-OH) and suppressed oxidative stress-induced injury in several organs[4],[5]. Oharazawa et al
[6] first reported that molecular hydrogen could immediately penetrate the vitreous body after the administration of H2-loaded eye drops, which effectively protected the retina from injury.
Hydrogen gas is the most structurally simple gas in nature and was previously considered to be a physiologically inert gas. Molecular hydrogen has been recognized to possess antioxidative effects since the first report in 1988 [7]. Hydrogen gas has been proven to have anti-inflammatory effects through neutralizing free radicals and reducing oxidative stress in many diseases, including parasite-induced liver inflammation, hepatic ischemia, reperfusion injury, neonatal hypoxia-ischemia, myocardial ischemia-reperfusion injury, lung injuries, pancreatitis, and type II diabetes[8]–[15]. The anti-inflammatory effects of hydrogen in various diseases have been attributed to four major molecular mechanisms: specific scavenging activity for hydroxyl radicals, scavenging activity for peroxynitrite, gene regulation and signal-modulating activities. The four mechanisms are not mutually exclusive and some of them may be causally associated with other mechanisms. However, further research is required to elucidate the detailed associations of the four mechanisms. Previous research was mostly focused on the first two mechanisms, and few studies have been performed on gene expression and signaling pathway regulation.
Studies have shown that microglia cells are central nervous system-resident phagocytic cells that function as brain macrophages[16]. Microglia cells are resident mononuclear cells required for neuronal homeostasis in the cen-tral nervous system and the retina. Microglia cells have been proven to be activated by diabetes, and cultured microglia cells have been extensively used to study microglial behavior[17], [18]. Lipopolysaccharides(LPS) has been widely used in a model to simulate infection. Toll-like receptor 4 (TLR4) is a pattern recognition receptor that plays an essential role in innate immunity by responding to LPS and initiating signaling cascades that culminate in proinflammatory gene expression. In the TLR4-initiated signaling pathway, Myd88 was found to play an essential role for all TLRs. Moreover, accumulating evidence has indicated that the LPS-induced TLR4 signaling pathway consists of a Myd88-dependent pathway and a Myd88-independent pathway[19]. In the Myd88-dependent pathway, Myd88 recruits IL-1 receptor-associated kinase (IRAK) to TLR4 upon stimulation. Phosphorylated IRAK-1, together with TRAF6, dissociates from the receptor, and TRAF6 then interacts with TAK1, TAB1, and TAB2. The complex of TRAF6, TAK1 and TAB1 induces the activation of TAK1. Activated TAK1 phosphorylates the IKK complex consisting of IKK-α/β and NEMO/IKKλ and thereby induces the activation of the transcription factor NF-κB and the release of inflammatory cytokines[19]. In recent years, numerous reports have provided evidence that miRNAs are involved in the regulation of immune responses, in part via establishing a link between TLR signaling, miRNA expression and the activation of inflammatory pathways[20]–[23]. LPS has been reported to be associated with several miRNAs, including miR-9, miR-21, miR-199, and miR-155, which were shown to target NF-κB, programmed cell death 4 (PDCD4), IKK-β, and SHIP1, respectively[24]–[28].
The anti-inflammatory effect of molecular hydrogen has been proven in numerous studies, and increasing evidence indicates that hydroxyl radical scavenging may not be the only mechanism underlying the protective effects of hydrogen in diseases in which oxidative stress plays a less important role in pathogenesis[29], [30]. A number of more recent investigations have reported that hydrogen might influence signal transduction[31], [32], suggesting that hydrogen may act as a novel signaling molecule. In our study, we determined the expression changes of three selected miRNAs using qRT-PCR and four LPS-induced TLR4-signaling pathway proteins using Western blotting. Our results suggest that molecular hydrogen might exert its anti-inflammatory effect through regulating these signal molecules.
MATERIALS AND METHODS
Materials
Microglia cells were obtained from the Chinese Academy of Typical Culture Collection Cell Bank (Shanghai, China). Fetal Bovine Serum (FBS) was obtained from Thermo Scientific (Hudson, NH, USA). Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) and miRVana™ miRNA extraction kit were obtained from Invitrogen (Carlsbad, CA). Antibiotic-Antimycotic mixture, trypsin-EDTA, and CCK-8 Cell Counting Kit were from HaiGene (Harbin, China). LPS and NF-κB1 antibody were purchased from Sigma-Aldrich (Shanghai, China). RT miRNA qPCR Assay Kit, the primers for miR-9, miR-21, miR-199 and control U6 were all purchased from Qiagen. RIPA lysis buffer, BCA Protein Assay Kit, HRP-conjugated secondary antibodies, β-actin antibody, Super ECL reagent, and the Super Signal Western Blot Kit were all obtained from HaiGene (Harbin, China). NC membrane was purchased from PALL(New York, USA). Myd88 antibody and PDCD4 antibody were obtained from Cell Signaling Technology. IKK-β antibody was from Beyotime (Haimen, China).
Methods
Cell culture and LPS treatment
Microglia cells were grown in DMEM/F12 medium containing 10% fetal bovine serum (FBS), 100U/mL of penicillin, and 100µg/mL of streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Microglia cells collected from culture flasks were seeded at a density of 3×105/well into 6-well tissue culture plates and cultured for 24 hours. One day after seeding, the culture wells were washed with PBS, and the cells were incubated at 37°C for 36 hours in 1mL serum-free medium in the presence or absence of 100ng/mL LPS. The cell survival rate was determined by CCK-8 cell counting kit according to the manufacturer’s instructions. After the LPS treatment, the LPS-containing medium was removed, the cells were washed twice with PBS, and hydrogen-saturated medium (prepared as described below) was added and cultured for 24 hours.
Cell hydrogen treatment
For hydrogen treatment of the cultured cells, we followed the procedure of Itoh et al[31]. The hydrogen concentration was measured using an H2-N hydrogen needle sensor (Unisense, Aarhus, Denmark). We calibrated the sensor with hydrogen- saturated water (1.6 ppm). The saturated water was prepared by bubbling 15mL of sterilized water with hydrogen gas for 30 minutes. We formulated a hydrogen-saturated DMEM/F12 medium through introducing hydrogen gas into the medium, and we calibrated the hydrogen concentration of the hydrogen-saturated medium (1.0×10−6). The hydrogen concentration of the medium 24 hours after hydrogen treatment was not less than 0.5×10−6. The hydrogen gas introducing was realized by humidified conditions of 75% H2, 20%O2 and 5%CO2. We dissolved H2 into DMEM/F12 medium under 0.5 MPa pressure, and O2 into a second medium by bubbling O2 gas at the saturated level (42mg/L), and CO2 into a third medium by bubbling CO2 gas. The three media were maintained at atmospheric pressure and then were combined in the proportion 75%:20%:5% (vol/vol/vol) and fetal bovine serum (FBS) was added to achieve a final concentration of 10%. We established two hydrogen treatment groups: the control+H2 group, in which hydrogen was introduced at a saturated level in the control medium (hydrogen-saturated DMEM/F12 medium); and the LPS+H2 group, in which the control medium was replaced with hydrogen-saturated DMEM/F12 medium after LPS treatment. Microglia cells of the two hydrogen treatment groups were cultured for 24 hours in a culture flask filled with the above combined medium. The CCK-8 Cell Counting Kit was used to determine the cell survival rate among different groups.
Small RNA isolation and miRNA qRT-PCR
Retinal microglia cells were divided into four groups: a control group (without any treatment), an LPS group (treated with 100ng/mL LPS), a control+H2 group (only treated with saturated hydrogen medium), and an LPS+H2 group (treated with saturated hydrogen medium after LPS treatment). Small RNAs were isolated using the miRVana™ miRNA extraction kit according to the manufacturer’s instructions. qRT-PCR was performed according to the manufacturer’s protocol of the RT miRNA qPCR Assay Kit. SYBR Green qRT-PCR was performed using 25ng of small RNA template under the following conditions: 95°C for 15 minutes and 40 cycles of 95°C for 10 seconds and 55°C for 30 seconds. For each sample, qRT-PCR was performed in quadruplicate. Each sample was normalized to the corresponding values of the U6 control.
Western blotting
Each group of retinal microglia cells was harvested after the corresponding treatment and lysed with Harsh RIPA lysis buffer according to the manufacturer’s instructions. The protein concentration was determined with a BCA Protein Assay Kit. Then, 20µg of the total protein lysate was analyzed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto NC membrane. The membrane was incubated with primary and HRP-conjugated secondary antibodies using the Super Signal Western Blotting Kit. The membranes were developed with Super ECL reagent and exposed to film. Each protein was normalized to the corresponding values of the β-actin control.
Statistical Analysis
All of the data were expressed as the mean±SD. The differences among groups were examined by a one-way analysis of variance (ANOVA). SPSS 13.0 software (SPSS Inc., Chicago, USA) was used to compare the differences among groups. P<0.05 was considered to be statistically significant.
RESULTS
Regulation of miR-9, miR-21 and miR-199 by Molecular Hydrogen
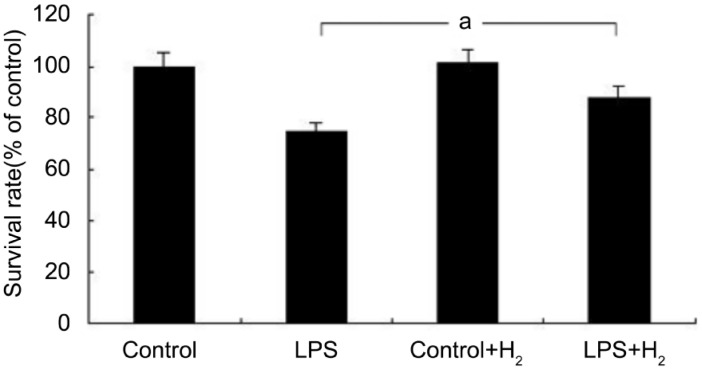
miR-9, miR-21 and miR-199 are strongly associated with the LPS-activated signaling pathway[25]–[27]. To investigate whether molecular hydrogen inhibited LPS-activated inflammation through regulating these miRNAs, we first detected the expression of these three miRNAs in retinal microglia cells treated with or without hydrogen. The retinal microglia cells were analyzed in four groups of treatment conditions: control, LPS, control+H2, and LPS+H2. We observed the partial apoptosis of microglia in the LPS group, and there were no significant changes in the control+H2 group, but the cell survival rate in the LPS+H2 group was higher than the LPS group using the CCK-8 method (Figure 1). qRT-PCR was conducted to detect changes in the expression of miR-9, miR-21, and miR-199. The results showed that miR-9 and miR-21 were decreased in the LPS+H2 group compared with LPS group (P<0.05), while miR-199 was increased (Figure 2). These data suggested that the anti-inflammatory effect of hydrogen might be strongly associated with changes in miRNA expression.
Regulation of miRNA Target Proteins by Hydrogen
To further understand the anti-inflammatory mechanism of molecular hydrogen’s effect on miRNA level, we detected the expression of selected miRNA target proteins, including NF-κB, PDCD4 and IKK-β using Western blotting. Because of the essential role of Myd88 in the LPS-activated TLR4-signaling pathway, the expression of Myd88 was also detected in the present study. The expression of Myd88 and IKK-β was increased after LPS treatment but significantly decreased after hydrogen treatment (Figure 3). The expression of PDCD4 was decreased by LPS treatment but significantly increased by molecular hydrogen. However, there was no significant change in NF-κB expression between LPS group and LPS+H2 group. These data suggested that molecular hydrogen not only scavenged hydroxyl radicals but also regulated the LPS-activated signaling pathway at the posttranscriptional level, including miRNAs and their target proteins (Figure 4).
A: Western Blot analysis of the protein express variation between LPS and H2 treatment, and β-actin served as a loading control; B: The histogram display of the band intensity was quantified using the Quantity One program, normalized relative to their respectiveβ-actin bands, and expressed as percentages of the control value.
DISCUSSION
Hydrogen gas, a potential antioxidant, has certain unique properties; unlike most known antioxidants, hydrogen is permeable to cell membranes and can target organelles, including mitochondria and nuclei. Its rapid gaseous diffusion might make it highly effective for reducing cytotoxic radicals. Moreover, hydrogen does not disturb the innate immune system and allows the phagocytosis of infecting organisms. In addition, H2 at low concentrations (<4%) is neither explosive nor dangerous. Therefore, hydrogen may become an ideal treatment choice for retinal injury in the clinic in the future.
In recent years, investigations have focused on the activation of microglia in diabetic retinopathy[18], [33]. Activated microglia have the potential of producing cytotoxic substances, such as TNF-α, reactive oxidative species, and proteases, which may induce neuronal degeneration[34]. Although the pathogenesis of diabetic retinopathy is not completely defined, accumulating evidence suggests that diabetic retinopathy exhibits many features of chronic inflammation, such as increased nitric oxide production, intracellular adhesion molecule-1 up-regulation, leukostasis, and the release of proinflammatory cytokines. It had also been shown that LPS activated microglia and exerted neurocytotoxic effects in vitro and in vivo[35], [36]. Therefore, it is feasible to use LPS-treated retinal microglia in culture to investigate the roles of microglia in retinal inflammation.
Based on basic and clinical studies, H2 has been indicated to be an important physiological regulatory factor with antioxidant, anti-inflammatory, and anti-apoptotic properties[37]. Previous research had focused on its antioxidant effect through neutralizing hydroxyl radicals [38], and only a few studies have reported the regulation of gene expression, such as TNFα, IL-1 and NF-κB and its signaling-modulating activity[9], [32]. Previous research had proved that miRNAs were involved in the regulation of the LPS-induced inflammatory signaling pathway. In the present study, we first determined the regulation of the expression of miRNAs and their target signaling-associated proteins in response to hydrogen. We found that the increased expression of miR-9 and miR-21 induced by LPS was down-regulated by hydrogen treatment, while miR-199 expression was up-regulated. Regarding the signaling-associated proteins, the increased expression of Myd88 and IKK-β induced by LPS was down-regulated by hydrogen treatment, while the decreased expression of PDCD4 was up-regulated, and there was no significant change in NF-κB expression.
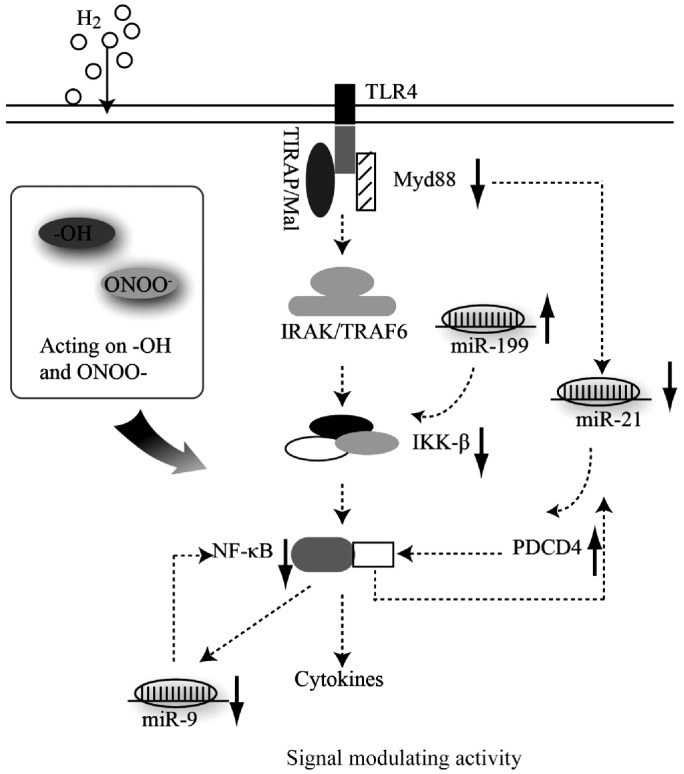
The LPS-activated inflammation signaling pathway is modulated by the activation of nuclear factor NF-κB and the release of inflammatory cytokines in a TLR4-Myd88-dependent and Myd88-independent manner. Moreover, a growing amount of evidence has indicated that miRNAs are involved in the regulation of LPS-induced inflammation through repressing gene expression at the post-transcriptional level. It has been reported that miR-9 induction by LPS depends on the activation of the Myd88 pathway and the identification of NF-κB consensus binding sites within the putative pri-miR-9-1 promoters suggested that miR-9 induction by LPS may result from the transcriptional activity of NF-κB [25]. Also it was proved that miR-9 postranscriptionally affected NF-κB specificly by renilla luciferase gene assay system[25]. Therefore it was deduced that NF-κB might be the direct target of miR-9 in LPS-induced signaling pathway. However, further more research is required to detect whether miR-9 regulates anti-inflammatory effect of hydrogen gas through NF-κB directly. It should be noted that there was no significant expression variation in NF-κB after hydrogen treatment compared with LPS treatment. A previous study showed that molecular hydrogen reduced epithelial apoptosis in ventilator-induced lung injury via activating NF-κB[39]. PDCD4 acts as a tumor suppressor upon apoptotic stimuli, and it was found that LPS modulated the expression of PDCD4 through the induction of miR-21, and this modulation regulated the activation of NF-κB[26]. IKK-β is the direct upstream activator of NF-κB, which is crucial for the production of proinflammatory cytokines, and IKK-β has been proven to be essential for the TNFα-induced activation of NF-κB. miR-199 was found to inhibit IKK-β mRNA translation through its 3’UTR in ovarian cancer[27]. Thus, it is possible that the LPS-induced signaling pathway is regulated by both signaling proteins and miRNAs, which provide feedback to each other. In the present study, it was demonstrated that the expression of miR-9, miR-21 and miR-199 and the expression of related signaling proteins were all influenced by hydrogen treatment at different degrees. Hydrogen treatment down-regulated the expression of miR-9 and miR-21, which further inhibited the activation of NF-κB, and the down-regulation of Myd88 inhibited the expression of miR-21, which activated PDCD4 to inhibit the NF-κB-induced release of inflammatory cytokines. The down-regulation of miR-199 after hydrogen treatment inhibited the activation of IKKβ to inhibit NF-κB, and the potential regulatory pathway is summarized in Figure 4, in which the activity of neutralizing -OH and scavenging ONNO− was also described. The different regulating mechanisms of hydrogen gas were not exclusive but associated with each other, such as promoting cell survival, altering gene expression, and regulating signaling-pathway activity. In summary, hydrogen treatment can inhibit inflammation through the regulation of miRNAs and signal-modulating activities, which suggests that hydrogen may act as a novel gas signaling molecular. It will be beneficial to understand the mechanism underlying this regulation and apply it to the clinic in the future.
Footnotes
Foundation items: Foundation of Heilongjiang Educational Committee Project, China (No. 12511313); Heilongjiang Provincial Foundation for Youths Project, China (No. QC2011C119); Research Program of Heilongjiang Provincial Health Office, China (No. 2009-197)
REFERENCES
The Original Article:
original title: Molecular hydrogen regulates the expression of miR-9, miR-21 and miR-199 in LPS-activated retinal microglia cells
Guo Liu, Hong Zhang, Lin Wang, Qing Han, Shi Zhou, Ping Liu
-
Abstract:
Aim: To explore the potential mechanism of molecular hydrogen in the regulation of miRNA expression and signal-modulating activities.
Methods: Retinal microglia cells were activated by Lipopolysaccharides (LPS) and then treated with hydrogen-saturated medium or normal medium without hydrogen. qRT-PCR was used to detect the expression difference in miR-9, miR-21 and miR-199 between these two groups. Moreover, the expression of LPS-induced signaling proteins, including Myd88, IKK-β, NF-κB, and PDCD4, were detected by Western blotting.
Results: The results demonstrated a marked down-regulation of miR-9 and miR-21 and up-regulation of miR-199 by hydrogen treatment; the expression of Myd88 and IKK-β was decreased after hydrogen treatment, whereas PDCD4 was increased, and there was no significant change in NF-κB expression.
Conclusion: The results in the present study indicate that miR-9, miR-199 and miR-21 play an important role in the anti-inflammatory regulation of LPS-activated microglia cells by molecular hydrogen, which will help to explain the protective mechanism of molecular hydrogen against inflammatory injury.