H2 and Hyperoxia Combo Improves Survival & Organ DamageScientific Research

Combination therapy of molecular hydrogen and hyperoxia improves survival rate and organ damage in a zymosan-induced generalized inflammation model
YUNCHUAN HONG
1Department of Respiratory Medicine, General Hospital of Tianjin Medical University, Tianjin 300052, P.R. China
LI SUN
2Department of Anesthesiology, General Hospital of Beijing Military Command, Beijing 100700, P.R. China
RUIQIANG SUN
3Department of Anesthesiology, Tianjin Eye Hospital, Tianjin Key Lab of Ophthalmology and Visual Science, Tianjin 300020, P.R. China
HONGGUANG CHEN
4Department of Anesthesiology, Tianjin Institute of Anesthesiology, General Hospital of Tianjin Medical University, Tianjin 300052, P.R. China
YONGHAO YU
4Department of Anesthesiology, Tianjin Institute of Anesthesiology, General Hospital of Tianjin Medical University, Tianjin 300052, P.R. China
KELIANG XIE
4Department of Anesthesiology, Tianjin Institute of Anesthesiology, General Hospital of Tianjin Medical University, Tianjin 300052, P.R. China
5Department of Anesthesiology, Clinical Medical School of Yangzhou University, Yangzhou, Jiangsu 225001, P.R. China
Abstract
Multiple organ dysfunction syndrome (MODS) is a leading cause of mortality in critically ill patients. Hyperoxia treatment may be beneficial to critically ill patients. However, the clinical use of hyperoxia is hindered as it may exacerbate organ injury by increasing reactive oxygen species (ROS). Hydrogen gas (H2) exerts a therapeutic antioxidative effect by selectively reducing ROS. Combination therapy of H2 and hyperoxia has previously been shown to significantly improve survival rate and organ damage extent in mice with polymicrobial sepsis. The aim of the present study was to investigate whether combination therapy with H2 and hyperoxia could improve survival rate and organ damage in a zymosan (ZY)-induced generalized inflammation model. The results showed that the inhalation of H2 (2%) or hyperoxia (98%) alone improved the 14-day survival rate of ZY-challenged mice from 20 to 70 or 60%, respectively. However, combination therapy with H2 and hyperoxia could increase the 14-day survival rate of ZY-challenged mice to 100%. Furthermore, ZY-challenged mice showed significant multiple organ damage characterized by increased serum levels of aspartate transaminase, alanine transaminase, blood urea nitrogen and creatinine, as well as lung, liver and kidney histopathological scores at 24 h after ZY injection. These symptoms where attenuated by H2 or hyperoxia alone; however, combination therapy with H2 and hyperoxia had a more marked beneficial effect against lung, liver and kidney damage in ZY-challenged mice. In addition, the beneficial effects of this combination therapy on ZY-induced organ damage were associated with decreased serum levels of the oxidative product 8-iso-prostaglandin F2α, increased activity of superoxide dismutase and reduced levels of the proinflammatory cytokines high-mobility group box 1 and tumor necrosis factor-α. In conclusion, combination therapy with H2 and hyperoxia provides enhanced therapeutic efficacy against multiple organ damage in a ZY-induced generalized inflammation model, suggesting the potential applicability of H2 and hyperoxia in the therapy of conditions associated with inflammation-related MODS.
Introduction
Multiple organ dysfunction syndrome (MODS) is the leading cause of mortality in critically ill patients (1). MODS is defined as the progressive deterioration of organ function, which often occurs in patients with severe sepsis, septic shock, hemorrhagic shock, multiple trauma, severe burns or pancreatitis (2). The mechanisms underlying the pathology of MODS are not fully understood (3), therefore it is difficult to develop an effective therapeutic measure for patients with MODS.
Oxygen inhalation is frequently administered to critically ill patients. Early goal-directed therapy for MODS aims to balance tissue oxygen delivery and oxygen demand. Previous studies have suggested that hyperoxia may have a beneficial effect against sepsis and sepsis-associated multiple organ damage (4–8). However, the clinical use of hyperoxia is limited in critically ill patients due to concerns that it may exacerbate organ damage by increasing free radical formation. Oxidative stress is crucially involved in the pathogenesis of MODS, and overproduction of reactive oxygen species (ROS) may exacerbate organ damage (9,10). It has been suggested that a low concentration of molecular hydrogen (H2) exerts a therapeutic antioxidative effect by selectively reducing hydroxyl (•OH)radicals, the most cytotoxic ROS, and peroxynitrite (ONOO−), and effectively protects against numerous diseases (11–15). Furthermore, our previous study showed that combination therapy with H2 and hyperoxia can significantly alleviate organ injury and improve survival rate of mice with polymicrobial sepsis via reducing oxidative stress and inflammation (16).
Therefore, these findings strongly indicate that combination therapy with H2 and hyperoxia may afford a more potent therapeutic strategy for sepsis. The zymosan (ZY)-induced generalized inflammation model has been widely used by other research groups (17), as well as by our group (8), because ZY, a substance derived from the cell wall of the yeast Saccharomyces cerevisiae, can produce systemic inflammation by inducing a wide range of inflammatory mediators (18). This model has also been used in previous experimental studies investigating MODS (18). Therefore, the aim of the present study was to investigate whether combination therapy with H2 and hyperoxia could produce enhanced efficacy in a murine model of ZY-induced generalized inflammation.
Materials and methods
Animals
Male Imprinting Control Region (ICR) mice (specific pathogen-free; age, 6–8 weeks; weight, 20–25 g) provided by the Laboratory Animal Center of the Academy of Military Science of the Chinese People’s Liberation Army (Beijing, China) were used in all experiments. Animals were housed at 20–22°C with a 12-h light/dark cycle. Animals were fed standard chow and water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University (Tianjin, China), and were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals (19).
ZY-induced generalized inflammation model
ZY (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in an isotonic sodium chloride solution (normal saline [NS]) to a final concentration of 25 mg/ml and was sterilized at 100°C for 80 min. All suspensions were freshly produced prior to use. Generalized inflammation was induced by an aseptic intraperitoneal injection of ZY (1 g/kg) (8,18). The same volume of NS was injected via the same route as a control.
Molecular hydrogen and/or hyperoxia treatment
The animals were put in a sealed plexiglass chamber with inflow and outflow outlets (20,21). H2, O2 or N2 was supplied via a gas flowmeter, (Yukata Engineering Corp., Tokyo, Japan) and delivered into the chamber through a tube at a rate of 4 l/min. The concentrations of O2 and H2 in the chamber were continuously monitored using a gas analyzer (LB-2, Model 40 M; Beckman Coulter, Inc., Fullerton, CA, USA) and a commercially available hydrogen detector (HY-ALERTA™ 500, H2scan Corporation, Valencia, CA, USA), respectively. CO2 was removed from the chamber gases using baralyme (Allied Healthcare Products, Inc., St. Louis, MO, USA). The mixed gases were maintained at the following levels: 2% H2, 21% O2 and 77% N22, 98% O2 and 2% N22, 98% O2 and 0% N2 during the treatment. The animals without H2 or hyperoxia treatment were exposed to room air in the chamber.
Experimental design
Experiment one: Effects of H2 and/or hyperoxia treatment on the survival rate in ZY-challenged mice
A total of 180 animals were randomly allocated into six groups (n=30 per group): Normal saline (NS), NS + H2 + O2222 + O2 groups. The treatment concentrations of hydrogen and hyperoxia were determined based on our previous studies and preliminary observations (8,21). The animals in the ZY + O2 and ZY + H2 groups were exposed to 98% O2 or 2% H2 for 3 h starting at 1 and 6 h after ZY injection, respectively. The animals in the NS + H2 + O2 and ZY + H2 + O2 groups were exposed to 98% O2 and 2% H2 at the same time points. As a control, the animals from the NS and ZY groups were given room air treatment at the same time points. The survival rate was observed on days 1, 2, 3, 4, 5, 6, 7 and 14 after NS or ZY injection. In addition, arterial blood gas evaluation was conducted at 1.5 h after the onset of H2 inhalation (2.5 h after NS or ZY injection) in all groups.
Experiment two: Effects of H2 and/or hyperoxia treatment on organ injury in ZY-challenged mice
Additional 36 animals were used in this experiment and were assigned to six groups (n=6 per group). The grouping method and experimental protocols were the same as Experiment One. At 24 h after NS or ZY injection, all the animals were sacrificed with sodium pentobarbital (50 mg/kg, intraperitoneally), and the blood samples and organs were collected for detecting serum biochemical parameters and organ histopathology.
In addition, at 24 h after NS or ZY injection, the serum levels of the proinflammatory cytokines high-mobility group box 1 (HMGB1) and tumor necrosis factor-α (TNF-α), the antioxidative enzyme superoxide dismutase (SOD) and the oxidative product 8-iso-prostaglandin F2α (8-iso-PGF2α) were evaluated.
Arterial blood gas analysis
The arterial blood gas analysis (for pH, PaO2 and PaCO2) was conducted using a GEM Premier 3000 gas analyzer (Instrumentation Laboratory Spa, Milan, Italy).
Serum biochemical parameters assay
The serum was separated from whole blood samples by centrifugation at 1,100 × g for 10 min, aliquoted and stored at −80°C until assayed (8,20–22). The samples were evaluated using a biochemistry autoanalyzer (7150; Hitachi, Ltd., Tokyo, Japan) to measure serum levels of alanine aminotransferase (ALT, IU/l), aspartate aminotransferase (AST, IU/l), blood urea nitrogen (BUN, mmol/l) and creatinine (Cr, µmol/l).
Organ histological examination
The lung, liver and kidneys were removed immediately, fixed in 4% paraformaldehyde (Sigma-Aldrich), embedded in paraffin, and sectioned at 4–6 µm. After deparaffinization and rehydration, the sections were stained with hematoxylin and eosin (Sigma-Aldrich). Based on the scoring standard in our previous studies (8,22), the histological slides were blindly examined and scored by two experienced pathologists.
Detection of SOD activity
The activities of SOD were measured using commercial kits purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Total SOD activity was assayed according to the manufacturer’s instructions and the protocol described in our previous studies (8,20–22). All spectrophotometric readings were performed using a spectrophotometer (DU-640B; Beckman Coulter, Inc.). All assays were conducted in triplicates.
Detection of 8-iso-PGF2α
Measurement of 8-iso-PGF2α, a free radical-catalyzed product of arachidonic acid, can offer a reliable approach for quantitative measurement of oxidative stress status in vivo (23). The levels of serum 8-iso-PGF2α were detected using specific enzyme-linked immunosorbent assay (ELISA) kits (cat. no. 516351; Cayman Chemical Company) and a Spectramax M5 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA) (8,20–22). All standards and samples were run in duplicate.
Detection of inflammatory cytokines
The levels of serum TNF-α (R&D Systems, Inc., Minneapolis, MI, USA) and HMGB1 (IBL International GmbH, Hamburg, Germany) were detected using specific ELISA kits and the Molecular Devices microplate reader (8,20–22). All standards and samples were run in duplicate.
Statistical analysis
Survival rates are expressed as percentages and measurement data are expressed as the mean ± standard error of the mean. The analysis of survival rates were tested using Fisher’s exact probability method. The inter-group differences of the rest data were tested by one-way analysis of variance followed by Least Significant Difference t-test for multiple comparisons. Statistical analyses were performed using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Differences in arterial blood gas during treatment
In the present study, the effects of H2 inhalation on arterial pH, PaO2 and PaCO2 were evaluated in all groups at 1.5 h after the onset of H2 and/or O2 inhalation (2.5 h after NS or ZY injection). The levels of PaO2 in the ZY + O2, ZY + H2 + O2 and NS + H2 + O2 groups were 405.1±19.2, 410.3±18.7 and 408.4±21.5 mmHg, respectively. The PaO2 levels in the NS, ZY and ZY + H2 groups were 97.18±8.29, 96.72±10.31 and 95.46±8.46 mmHg, respectively. There were no differences in the levels of arterial pH and PaCO2 among all groups (data not shown). The results demonstrate that hyperoxia exposure can significantly increase the PaO2 level, and 2% H2 has no marked effects on the PaO2 level in ZY-challenged mice during the treatment.
Combination therapy with H2 and hyperoxia improves the survival rate in ZY-challenged mice
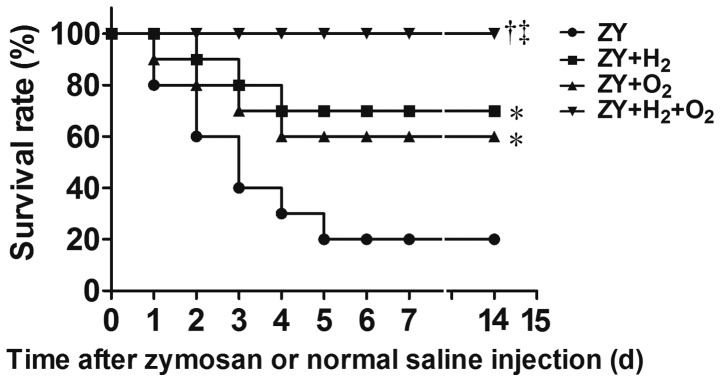
The 14-day survival rate of ZY-challenged mice was 20% (P<0.05 vs. NS group; Fig. 1). The results showed that either 98% O2 or 2% H2 exposure for 3 h starting at 1 and 6 h after ZY injection, respectively, improved the 14-day survival rate of ZY-challenged mice to 60 or 70% (P<0.05 vs. ZY group; Fig. 1). Furthermore, combination therapy with 2% H2 and 98% O2 increased the 14-day survival rate of ZY-challenged mice to 100% (P<0.05 vs. ZY group; Fig. 1). In addition, all mice in the NS and NS + H2 + O2 groups survived during the experiment. These results suggest that combination therapy with H2 and hyperoxia can improve the survival rate of ZY-challenged mice in a synergistic manner.
Effects of H2 and/or hyperoxia on the survival rate of zymosan-challenged mice. The mice were treated with or without 2% H2 and/or 98% O2 inhalation for 3 h starting at 1 and 6 h after normal saline or zymosan injection, respectively. Values are expressed as survival percentage (n=30 per group). *P<0.05 vs. ZY group; †P<0.05 vs. ZY + H2 group; ‡P<0.05 vs. ZY + O2 group. ZY, zymosan; d, days.
Combination therapy with H2 and hyperoxia improves organ histopathological scores in ZY-challenged mice
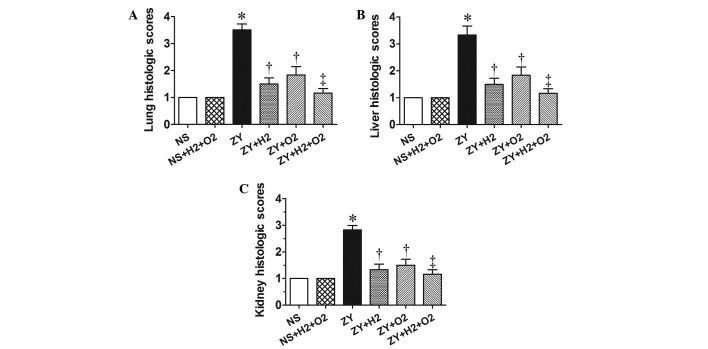
At 24 h after ZY or NS injection, the animals in all groups were killed for histopathological analysis. According to the scoring standard in our previous studies (8,22), the histopathological changes in lung, liver and kidney were scored using a scale of 1 to 4. As shown in the Fig. 2, the histopathological scores for lung, liver and kidney in the ZY group were 3–3.5, significantly increased compared with the NS group (P<0.05). These abnormal changes in ZY-challenged mice were attenuated by either 98% O2 or 2% H2 treatment alone (P<0.05 vs. ZY group; Fig. 2). Furthermore, these abnormal changes in ZY-challenged mice were more markedly ameliorated by combination therapy with 98% O2 and 2% H2 compared with either treatment alone (Fig. 2). These data indicate that combination therapy with H2 and hyperoxia has an enhanced efficacy against multiple organ damage in ZY-challenged mice.
Effects of H2 and/or hyperoxia on the organ histopathological scores in zymosan-challenged mice. Histological scores in the mouse (A) lungs, (B) liver and (C) kidneys. Mice were treated with or without 2% H2 and/or 98% O2 inhalation for 3 h starting at 1 and 6 h after NS or zymosan injection, respectively. At 24 h after NS or zymosan injection, all animals were anesthetized, and the organ samples were collected for measuring the histopathological scores. Values are expressed as the mean ± standard error of the mean (n=6 per group). *P<0.05 vs. NS group; †P<0.05 vs. ZY group; ‡P<0.05 vs. ZY + O2 group. NS, normal saline; ZY, zymosan.
Combination therapy with H2 and hyperoxia improves serum biochemical parameters in ZY-challenged mice
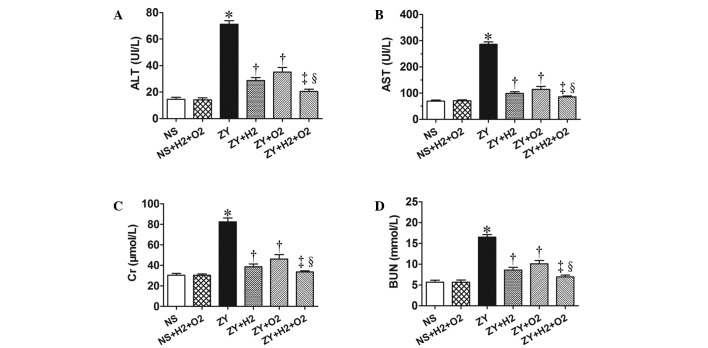
As shown in Fig. 3, the ZY-challenged mice appeared significantly impaired liver and kidney function at 24 h, which was assessed by serum biochemical parameters for liver and kidney function (ALT, AST, Cr and BUN). The ZY-challenged mice showed a significant increase in the levels of serum ALT, AST, Cr and BUN (P<0.05 vs. NS group), which were significantly attenuated by 2% H2 or 98% O2 treatment alone (Fig. 3). Furthermore, these abnormal changes of biochemical parameters in ZY-challenged mice were more notably ameliorated by combination therapy with 98% O2 and 2% H2 (Fig. 3). These results demonstrate that combination therapy with H2 and hyperoxia has a more substantially beneficial effect on liver and kidney dysfunction in ZY-challenged mice.
Effects of H2 and/or hyperoxia on the serum biochemical parameters in zymosan-challenged mice. Serum levels of (A) ALT, (B) AST, (C) Cr and (D) BUN. Mice were treated with or without 2% H2 and/or 98% O2 inhalation for 3 h starting at 1 and 6 h after NS or zymosan injection, respectively. At 24 h after NS or zymosan injection, all the animals were anesthetized, and the blood samples were collected for detection of the serum biochemical parameters. Values represent the mean ± standard error of the mean (n=6 per group). *P<0.05 vs. NS group; †P<0.05 vs. ZY group; ‡P<0.05 vs. ZY + H2 group; §P<0.05 vs. ZY + O2 group. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; UI/L, international units per liter; NS, normal saline; ZY, zymosan.
Combination therapy with H2 and hyperoxia prevents the abnormal changes of antioxidant enzymatic activities, oxidative product and inflammatory cytokines in ZY-challenged mice
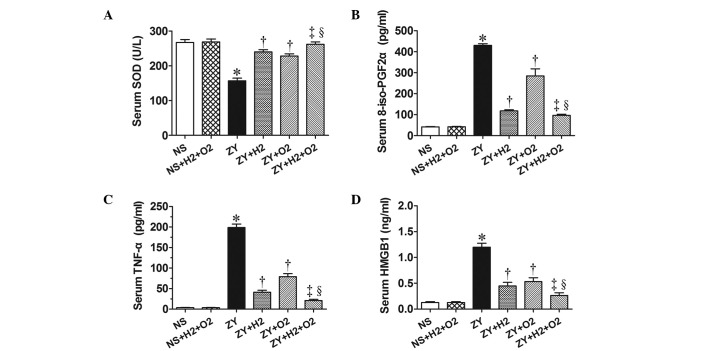
The activity of the antioxidative enzyme SOD, the levels of the oxidative product 8-iso-PGF2α and the levels of proinflammatory cytokines (HMGB1 and TNF-α) in serum of all animals were observed at 24 h after NS or ZY injection. The results showed decreased SOD activity and increased levels of 8-iso-PGF2α, HMGB1 and TNF-α in the serum of the ZY-challenged mice (P<0.05 vs. NS group; Fig. 4). Furthermore, the results showed that 2% H2 or 98% O2 alone significantly increased the SOD activity and decreased the 8-iso-PGF2α level in serum of ZY-challenged mice (P<0.05; Fig. 4). In addition, 2% H2 or 98% O2 alone significantly decreased the serum HMGB1 and TNF-α levels of the ZY-challenged mice (P<0.05; Fig. 4). These abnormal changes in oxidative stress and inflammatory cytokines were more markedly improved by combination therapy with 98% O2 and 2% H2 (Fig. 4).
Effects of H2 and/or hyperoxia on the activity of serum antioxidant enzyme as well as the levels of serum oxidative product and inflammatory cytokines in zymosan-challenged mice. (A) SOD activity and serum levels of (B) 8-iso-PGF2α, (C) TNF-α and (D) HMGB1 level. The mice were treated with or without 2% H2 and/or 98% O2 inhalation for 3 h starting at 1 and 6 h after NS or zymosan injection, respectively. The serum was harvested for measuring these indicators at 24 h after NS or zymosan injection. Values are expressed as the mean ± standard error of the mean (n=6 per group). *P<0.05 vs. NS group; †P<0.05 vs. ZY group; ‡P<0.05 vs. ZY + H2 group; §P<0.05 vs. ZY + O2 group. SOD, superoxide dismutase; 8-iso-PGF2α, 8-iso-prostaglandin F2α; HMGB1, high-mobility group box 1 protein; NS, normal saline; ZY, zymosan.
These data suggest that combination therapy with H2 and hyperoxia produces a more beneficial effect on survival rate and organ damage in ZY-challenged mice, which is associated with decreased levels of oxidative product and proinflammatory cytokines and increased levels of antioxidative enzymes in the serum.
Discussion
The present study demonstrated that either H2 or hyperoxia treatment alone improved the survival rate of ZY-challenged mice, while combination therapy with H2 and hyperoxia could synergistically increase the survival rate of ZY-challenged mice, which was greater than treatment with either gas alone. Furthermore, H2 or hyperoxia treatment alone protected against lung, liver and kidney damage in ZY-challenged mice, while combination therapy with H2 and hyperoxia provided cumulative protection against these organ damage of ZY-challenged mice. In addition, the beneficial effects of combination therapy with H2 and hyperoxia on ZY-induced organ injury were associated with decreased levels of oxidative product 8-iso-PGF2α, increased SOD activity and reduced levels of inflammatory cytokines TNF-α and HMGB1 in serum.
ZY is a substance derived from the cell wall of the yeast Saccharomyces cerevisiae, and can induce systemic inflammation by inducing a wide range of inflammatory mediators (18). Based on previous studies by the present authors and others, intraperitoneal injection of a high dose of ZY (0.8–1.0 g/kg) can induce a generalized inflammation in rats or mice, which is accompanied by multiple organ damage (8,17,18,22). ZY (1.0 g/kg, intraperitoneal injection) successfully induced sterile inflammation model in mice, characterized by the decrease of survival rates, histopathological injury, organ dysfunction and abnormally decreased tissue oxygenation (8,22). In the present study, similar changes were observed in the ZY-challenged mice.
Oxygen therapy is widely used in clinical practice as a mainstay of supportive treatment for patients with hypoxemia and critical illness. It is well known that early goal-directed therapy for MODS aims to balance oxygen delivery and demand (24,25). Previous animal studies have shown that hyperoxia exposure can improve organ function and survival rate in several models of shock or sepsis (4–8,26,27). In addition, 100% oxygen exposure for 2 and 3 h starting at 4 and 12 h, respectively, after ZY injection benefits the outcome of mice with sterile sepsis (8). It is speculated that improved tissue oxygenation and decreased systemic inflammatory response are crucially involved in the protective effects of hyperoxia treatment (4–8). However, hyperoxia treatment can induce the production of ROS, which are considered to be associated with oxygen toxicity (9,10). Therefore, the use of hyperoxia is limited in critically ill patients due to concerns that it may exacerbate organ damage by increasing free radical formation.
H2 has been used medically to prevent decompression sickness in deep divers for safety profiles (28). Prior studies have shown that H2 exerts a therapeutic antioxidative effect by selectively reducing ROS toxicity, and effectively protecting against a number of diseases, suggesting that H2 has potential as an antioxidant for therapeutic applications (11–15). Our previous studies have shown that H2 treatment has a beneficial effect on sepsis and sepsis-induced organ injury (16,20,21). In the present study, combination therapy with H2 and hyperoxia appeared to produce a synergistic protective effect against sepsis and sepsis-associated multiple organ damage (16). The present results showed that combination therapy with H2 and hyperoxia had a more beneficial effect on multiple organ dysfunction/failure in the ZY-induced generalized inflammation model.
To further investigate the possible underlying mechanisms, the effects of H2 and/or hyperoxia treatment on oxidant and antioxidant system in ZY-challenged mice were investigated. In our previous studies, we found that the activities of SOD, CAT and GSH-Px in serum and tissues are significantly decreased during the early and late stages of ZY-induced organ damage, indicating that ZY sets up an environment favorable for oxidative stress (8,22). In the present study, decreased SOD activity and increased 8-iso-PGF2α levels were detected in the serum at 24 h after ZY injection. Furthermore, 2% H2 and/or 98% O2 treatment significantly improved SOD activity and decreased the 8-iso-PGF2α levels in the serum. These results suggest that the reduction of oxidative damage and the increase of endogenous antioxidant enzymatic activities in the serum may associated with the protective effects of H2 and/or O2 treatment, which is consistent with our previous study (20). It has been hypothesized that the uncontrolled and exaggerated inflammatory response plays a major role in the pathogenesis of sepsis/MODS (3). Inflammatory cytokines include early inflammatory cytokines such as proinflammatory cytokines TNF-α, IL-6 and anti-inflammatory cytokine IL-10, as well as the late inflammatory cytokine HMGB1 (29,30). Early and late inflammatory cytokines can interact and facilitate the organ dysfunction and injury in sepsis/MODS. Our previous studies have demonstrated that HMGB1 contributed to organ damage in the ZY-induced generalized inflammation model (8,22). In the present study, we found that ZY-challenged mice showed the significant increase of TNF-α and HMGB1 in serum, which was significantly attenuated by 2% H2 and/or O2 treatment. These data suggest that the protective effects of combination therapy with H2 and hyperoxia treatment on ZY-challenged mice are associated with a reduction in the serum levels of early and late proinflammatory cytokines, which is consistent with our previous study (20,21).
H2 is neither explosive nor dangerous at a low concentration (<4.1% in oxygen). The present results suggest that a mixed gas therapy with H2 and hyperoxia may be a novel, safe and effective approach for preventing organ damage and mortality in MODS.
Acknowledgements
This study was supported by research grants from the National Natural Science Foundation of China (grant nos. 81101409 and 81471842) and Natural Science Foundation of Tianjin, China (no. 13JCQNJC11400), the Foundation of Tianjin Bureau of Public Health (no. 2011KZ108).
Glossary
Abbreviations
| 8-iso-PGF2α | 8-iso-prostaglandin F2α |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BUN | blood urea nitrogen |
| CAT | catalase |
| CLP | cecal ligation and puncture |
| Cr | creatinine |
| GSH-Px | glutathione peroxidase |
| H2 | hydrogen |
| H2O2 | hydrogen peroxide |
| HMGB1 | high-mobility group box 1 |
| ICU | intensive care units |
| MODS | multiple organ dysfunction syndrome |
| NS | normal saline |
| •OH | hydroxyl radicals |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| ZY | zymosan |
References
The Original Article:
original title: Combination therapy of molecular hydrogen and hyperoxia improves survival rate and organ damage in a zymosan-induced generalized inflammation model
Yunchuan Hong, L Sun, Ruiqiang Sun, Hongguang Chen, Yonghao Yu, Ke Xie
-
Abstract:
Multiple organ dysfunction syndrome (MODS) is a leading cause of mortality in critically ill patients. Hyperoxia treatment may be beneficial to critically ill patients. However, the clinical use of hyperoxia is hindered as it may exacerbate organ injury by increasing reactive oxygen species (ROS). Hydrogen gas (H2) exerts a therapeutic antioxidative effect by selectively reducing ROS. Combination therapy of H2 and hyperoxia has previously been shown to significantly improve survival rate and organ damage extent in mice with polymicrobial sepsis. The aim of the present study was to investigate whether combination therapy with H2 and hyperoxia could improve survival rate and organ damage in a zymosan (ZY)-induced generalized inflammation model. The results showed that the inhalation of H2 (2%) or hyperoxia (98%) alone improved the 14-day survival rate of ZY-challenged mice from 20 to 70 or 60%, respectively. However, combination therapy with H2 and hyperoxia could increase the 14-day survival rate of ZY-challenged mice to 100%. Furthermore, ZY-challenged mice showed significant multiple organ damage characterized by increased serum levels of aspartate transaminase, alanine transaminase, blood urea nitrogen and creatinine, as well as lung, liver and kidney histopathological scores at 24 h after ZY injection. These symptoms where attenuated by H2 or hyperoxia alone; however, combination therapy with H2 and hyperoxia had a more marked beneficial effect against lung, liver and kidney damage in ZY-challenged mice. In addition, the beneficial effects of this combination therapy on ZY-induced organ damage were associated with decreased serum levels of the oxidative product 8-iso-prostaglandin F2α, increased activity of superoxide dismutase and reduced levels of the proinflammatory cytokines high-mobility group box 1 and tumor necrosis factor-α. In conclusion, combination therapy with H2 and hyperoxia provides enhanced therapeutic efficacy against multiple organ damage in a ZY-induced generalized inflammation model, suggesting the potential applicability of H2 and hyperoxia in the therapy of conditions associated with inflammation-related MODS.