H2 gas prevents liver injury by inhibiting JNK activationScientific Research

Hydrogen gas alleviates acute alcohol-induced liver injury by inhibiting JNK activation
Yaxing Zhang
1Department of Traditional Chinese Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510630, P.R. China
Mingmin Bi
2Department of Otorhinolaryngology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong 518107, P.R. China
3Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong 510080, P.R. China
Zifeng Chen
4Biofeedback Laboratory, Xinhua College of Sun Yat-sen University, Guangzhou, Guangdong 510520, P.R. China
5Department of Biomedical Engineering, Xinhua College of Sun Yat-sen University, Guangzhou, Guangdong 510520, P.R. China
Min Dai
1Department of Traditional Chinese Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510630, P.R. China
Ge Zhou
1Department of Traditional Chinese Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510630, P.R. China
Yuxuan Hu
1Department of Traditional Chinese Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510630, P.R. China
Hongzhi Yang
1Department of Traditional Chinese Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510630, P.R. China
Weibing Guan
1Department of Traditional Chinese Medicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510630, P.R. China
Associated Data
- Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abstract
Binge alcohol drinking is fast becoming a global health concern, with the liver among the first organ involved and the one afflicted with the greatest degree of injury. Oxidative stress, alterations in hepatic metabolism, immunity and inflammation have all been reported to contribute to the development of alcoholic liver disease (ALD). Hydrogen gas (H2) serves a key role in the modulation of hepatic redox, immune and inflammatory homeostasis. However, the effects of treatment using intraperitoneal injection of H2 on ALD remain unexplored. Therefore, the aim of the present study was to investigate the effects and underlying mechanism of intraperitoneal injection of H2 on acute alcohol-induced liver injury in a mouse model. H2 was administered by daily intraperitoneal injections (1.0 ml/100 g) for 4 days. On day 4, the mice received H2 after fasting for 5.5 h. After 30 min, the mice were administered with 33% (v/v) ethanol at a cumulative dose of 4.5 g/kg body weight by four equally divided gavages at 20-min intervals. Blood and liver tissues were collected at 16 h after the first ethanol gavage. Subsequently, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride and total cholesterol (TC) levels were analyzed using an Automatic Clinical Analyzer. Hepatic JNK activity and GAPDH levels were examined by western blotting. It was observed that acute ethanol gavage induced liver injury, as indicated by significantly increased serum ALT and AST levels, which were effectively decreased by H2 at 16 h after the first ethanol gavage. In addition, H2 treatment reduced serum TC levels in the Alcohol+H2 group when compared with those in Alcohol group. Mechanistically, H2 attenuated hepatic JNK phosphorylation induced by acute ethanol gavage. Therefore, the results of the present study demonstrated that treatment with exogenous H2 by intraperitoneal injection may alleviate acute alcohol-induced liver injury by inhibiting hepatic JNK activation, which may represent a novel therapeutic strategy for ALD.
Introduction
Excessive alcohol intake, as result of binge drinking as well as chronic alcohol consumption of >40 g per day, is becoming a global healthcare concern (1,2). Globally, ~2 billion individuals consume alcohol regularly, where >75 million are diagnosed with disorders associated with alcohol abuse and are at risk of developing alcohol-associated liver diseases (3). The liver typically sustains the earliest and the greatest degree of tissue injury caused by excessive drinking, since it is the primary site of ethanol metabolism (1,4). Excessive alcohol consumption causes alcoholic liver disease (ALD), which is characterized by a wide spectrum of hepatic pathologies, from reversible fatty liver (simple steatosis) to acute alcoholic hepatitis, chronic fibrosis and cirrhosis and superimposed hepatocellular carcinoma (HCC) (2,5,6). Mechanistically, pathogenesis of ALD includes genetic susceptibility, oxidative stress, hepatic metabolism alteration and steatosis, acetaldehyde-mediated toxicity, cytokine- and chemokine-induced inflammation, alterations in immunity and dysbiosis, epigenetic changes and modifications in the regenerative process (2,6-8). Alcohol abstinence achieved by psychosomatic intervention is at present the best treatment for all stages of ALD (2). However, drinking discontinuation may be difficult, for example, the cheap price of hard liquor, easy accessibility to alcohol, and alcohol advertisement make it very difficult to prevent the increase in ALD (9). Therefore, new strategies for alleviating alcohol-induced liver injury remain to be in demand.
Hydrogen gas (H2) is the lightest and diffusible gas molecule that has been shown to confer potent antioxidant and anti-inflammatory properties (10,11). It can be absorbed into the blood circulation, such that it reaches the target organ either by blood circulation or free diffusion (11). Therefore, treatments involving exogenous H2, including breathing H2 gas, injection with H2-rich saline and drinking H2-rich water, may protect against excessive oxidative stress- and inflammation-related liver damage, including liver injury induced by drugs, sepsis, bile duct ligation, ischemia/reperfusion (I/R), CO2 pneumoperitoneum and chronic intermittent hypoxia, in addition to non-alcoholic fatty liver disease (NAFLD) (12-17). Furthermore, drinking H2-rich water has been indicated to protect against chronic ethanol-induced hepatotoxicity (18). These previous observations suggest that H2 serves an important role in modulating hepatic redox, immune and inflammatory homeostasis (11).
Previous studies have demonstrated that supplementation with exogenous H2 by intraperitoneal injection may improve lipopolysaccharide (LPS)-induced cardiac dysfunction, isoproterenol-induced cardiac hypertrophy, pressure overload-induced vascular hypertrophy, and cardiopulmonary cerebral resuscitation in a cardiac arrest rabbit model (19-23). These were mediated by the suppression of excessive oxidative stress and inflammatory responses (19-23). However, the effects of intraperitoneal injection of H2 on ALD remain unclear.
Therefore, the aim of the present study was to investigate the effect of H2 intraperitoneal injection on acute alcohol-induced liver injury in a mouse model and to elucidate the potential underlying mechanisms.
Materials and methods
Drugs
In total, 99,999% H2 (Dalian Special Gases Co., Ltd.) was injected into a vacuumed aseptic soft plastic infusion bag (100 ml; Hebei Tiancheng Pharmaceutical Co., Ltd.) from a seamless steel gas cylinder under sterile conditions, as previously described (Fig. 1) (20-23). Anhydrous ethanol (Guangzhou Chemical Reagent Factory) was dissolved in double-distilled water to obtain 33% (v/v) ethanol (0.26 g/ml) (24).
Preparation of H2 in the storage container. (A) Sterile saline and the gases in the aseptic soft plastic infusion bag (100 ml) were emptied using a 50-ml syringe under sterile conditions. (B) The gas outlet of seamless steel gas cylinder was connected to a silica gel tube, which was in turn connected to a 1-ml syringe needle at the other end. This syringe needle was inserted into the vacuumed plastic bag, which was filled by opening the reducing valve of the H2 steel cylinder until the bag was full of H2 gas with no dead volume.
Animal model of acute alcohol-induced liver injury and treatment protocol
Male C57BL/6J mice (Animal license no. 44007200061823) were purchased from the Guangdong Medical Laboratory Animal Center (Foshan, China). A total of 28 mice aged 8-10 weeks were used in this study. All animals were housed in a temperature-controlled animal facility (21-24˚C) with a 12-hour light-dark cycle, and the animals had access to rodent chow and water ad libitum (20). All mice were provided with humane care according to the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the NIH (8th Edition, Revised 2011) (20,25). All animal procedures were approved by the Institutional Animal Care and Use Committee of Zhongshan School of Medicine, Sun Yat-sen University (Guangzhou, China).
Mice were randomly assigned into the following four groups (n=7 mice per group): i) Control; ii) Alcohol; iii) Alcohol+H22. In the Alcohol+H2 and H2 groups, H2 was administered daily at the dose of 1.0 ml/100 g by intraperitoneal injection for 4 days. On day 4, mice in each group were fasted for 6 h before the mice in the Alcohol and Alcohol+H2 groups were orally administered with 33% (v/v) ethanol at a cumulative dose of 4.5 g/kg body weight (17.3 ml/kg body weight) by four equally divided gavages at 20-min intervals (24). Mice in the Alcohol+H2 and H2 groups were administered with an intraperitoneal injection (1.0 ml/100 g) of H2 30 min before the first ethanol administration (20). In addition, mice in the Control and H2 groups received the same volume of double-distilled water (17.3 ml/kg body weight) by four equally divided gavages at 20 min intervals. The animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) after 16 h of the first ethanol gavage, cardiac puncture was performed when the animals reached the surgical plane of anesthesia and ~0.4-0.5 ml blood was collected from each mouse (26-28). Euthanasia was then performed by cervical dislocation in a state of deep anesthesia before liver tissues were extracted (Fig. 2) (24). The body weight of animals in each group was recorded at the baseline (Control, 25.19±1.58 g; Alcohol, 24.63±1.42 g; Alcohol+H2, 25.17±1.15 g; H2, 23.9±0.70 g) and after intervention (Control, 24.90±1.36 g; Alcohol, 25.16±1.53 g; Alcohol+H2, 25.00±0.93 g; H2, 23.71±0.63 g).
Experimental procedures. On days 1-3, mice in the Alcohol+H2 and H2 groups were intraperitoneally injected with H2 (1.0 ml/100 g) at 18:00 daily. On day 4, the mice were fasted starting at 12:00 for 6 h before the first ethanol administration. Mice in the Alcohol+H2 and H2 groups received H2 at 17:30. Mice in the Alcohol and Alcohol+H2 groups were orally administered 33% (v/v) ethanol at an accumulative dosage of 4.5 g/kg body weight using four equally divided gavages at 18:00, 18:20, 18:40 and 19:00. Mice in the Control and H2 groups received the same volume of ddH2O. On Day 5, the mice were anesthetized and euthanized for blood and tissue collection at 10:00.
Biochemical analysis
Blood biochemical analysis was performed as previously described (29). Briefly, the blood samples were centrifuged at the speed of 986 x g for 10 min at 4˚C to separate the serum. Levels of liver function markers alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in addition to the levels of lipid markers triglyceride (TG) and total cholesterol (TC) in the serum were analyzed using an Automatic Clinical Analyzer (Hitachi 7600; Hitachi High-Technologies Corporation) at the Department of Clinical Laboratory, The Third Affiliated Hospital of Sun Yat-sen University.
Western blotting
JNK antibody (cat. no. 9252S), phosphorylated (p-) JNK antibody (cat. no. 9255S), anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. 7074S) and anti-mouse IgG HRP-conjugated secondary antibody (cat. no. 7076S) were purchased from Cell Signaling Technology, Inc. GAPDH antibody (cat. no. MB001) was obtained from Bioworld Technology, Inc. BSA (cat. no. ST023-200g) was purchased from Beyotime Institute of Biotechnology.
Western blotting was performed as previously described (30). The proteins were transferred onto polyvinylidene fluoride membranes (EMD Millipore). Membranes were incubated in blocking buffer (1X TBST with 5% BSA) for 30 min at room temperature. For antibody incubations, membranes were incubated with primary antibodies in antibody dilution buffer (1X TBST with 5% BSA) with gentle agitation overnight at 4˚C (p-JNK and JNK: 1:2,000; GAPDH: 1:10,000), and with secondary antibodies in antibody dilution buffer (1X TBST with 5% BSA) with gentle agitation for 1 h at room temperature (anti-rabbit IgG HRP-conjugated secondary antibody and anti-mouse IgG HRP-conjugated secondary antibody: 1:2,000). Immobilon™ Western Chemiluminescent HRP Substrate (ECL; cat. no. WBKLS0100, EMD Millipore) was used to reveal the bands using the ChemiDoc™ Touch Imaging System (Bio-Rad Laboratories, Inc.). Image J software (Version 1.52; National Institutes of Health) was used for estimating the ‘IntDen’ value of the western blot bands for quantification.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc.). Data were expressed as the mean ± SD. For biochemical analysis, n=7 mice in each group. For western blotting, n=5 mice in each group. Statistical analysis was performed by one-way ANOVA followed by Bonferroni’s post hoc test. P<0.05 was considered to indicate statistically significant differences.
Results
H2 treatment prevents acute alcohol-induced liver damage
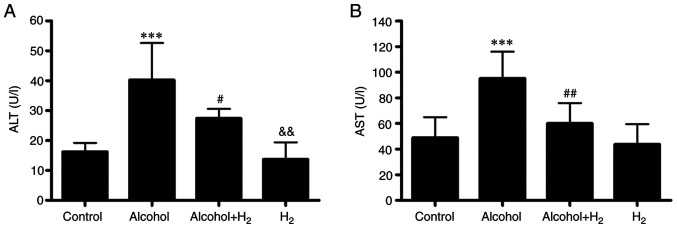
To investigate the effects of H2 on alcohol-induced liver injury, an acute alcohol-induced hepatotoxicity mouse model was established as previously described (24). This model was shown to closely mimic excessive ethanol consumption in humans in terms of blood alcohol levels, behavioral and physiological effects (24). Compared with those in the Control group, serum ALT and AST levels in the Alcohol group were significantly higher following acute alcohol gavage (Fig. 3), whilst intraperitoneal injection of H2 significantly prevented these elevations in serum ALT and AST levels in mice following alcohol gavage (Fig. 3). However, H2 alone exerted no effects on serum ALT and AST levels compared with those in the Control group (Fig. 3). Therefore, these observations suggest that intraperitoneal injection of H2 effectively alleviated acute alcohol-induced liver injury in mice.
H2 treatment reduces serum ALT and AST levels after ethanol feeding. Serum (A) ALT and (B) AST levels. n=7 mice in each group. ***P<0.001 vs. Control and vs. H2#P<0.05 vs. Control and vs. Alcohol; ##P<0.01 vs. Alcohol; &&P<0.01 vs. Alcohol+H2. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Effects of H2 on serum lipid levels in acute alcohol-induced liver injury
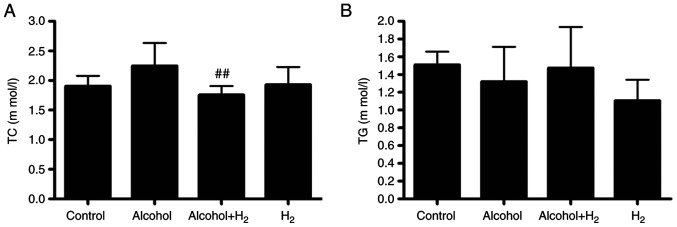
Long-term chronic alcohol consumption may lead to disruptions in lipid metabolism (6). Therefore, the serum levels of TC and TG were next examined. Although acute alcohol treatment slightly increased serum TC levels, there was no significant difference between those in the Control and Alcohol groups (Fig. 4A). Serum TC levels in the Alcohol+H2 group were significantly lower compared with those in the Alcohol group (Fig. 4A). However, there were no significant differences in TG levels among the four groups (Fig. 4B). These data suggest that intraperitoneal injection of H2 may modulate blood TC levels in an acute alcohol-induced liver injury mice model.
H2 alleviates acute alcohol-induced hepatic JNK activation
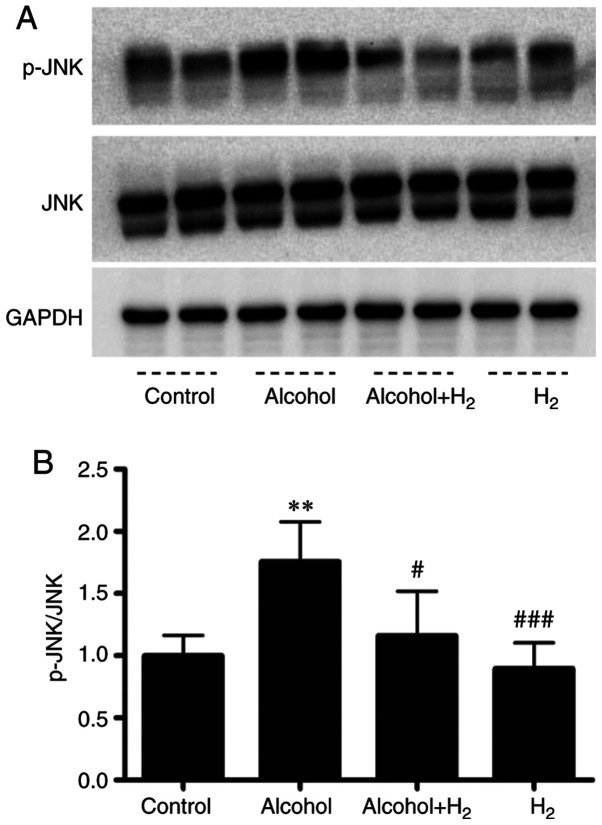
Acute ethanol loading causes oxidative stress and activates cell-death signaling through the JNK pathway in the liver (31). In addition, JNK is a key mediator in hepatic steatosis, where it regulates transcription factor activity associated with lipid metabolism (32,33). In the present study, the upregulation of serum ALT and AST levels after ethanol treatment indicated hepatocytes damage. Therefore, the role of hepatic JNK activation in the potentially protective effects of H2 against acute alcohol-induced liver injury was examined. Western blotting data demonstrated that acute ethanol treatment significantly increased hepatic JNK phosphorylation, which was significantly prevented by intraperitoneal injection with H2 (Fig. 5). Therefore, these data suggest that intraperitoneal injection with H2 conferred protective effects against acute alcohol-induced liver injury by preventing hepatic JNK activation.
Effects on H2 on hepatic JNK activation after ethanol feeding. (A) Representative western blotting images of p-JNK and JNK and GAPDH. (B) Quantification of p-JNK/total JNK ratio. The averages of the p-JNK/JNK ratio in the Control group were set as ‘1’, and all values were normalized to ‘1’. n=5 mice in each group. **P<0.01 vs. Control; #P<0.05 and ###P<0.001 vs. Alcohol.
Discussion
Inflammatory and cytokine signaling, oxidative stress and mitochondrial dysfunction, alterations in hepatic metabolism and hepatocyte cell death, and abnormalities in immunity and dysbiosis are all key to the pathogenesis of liver diseases, including ALD and NAFLD (2,34-36). Therefore, strategies preventing fatty liver disease progression were previously developed using combinations of naturally occurring compounds products in animal models. For example, the combination of docosahexaenoic acid and the antioxidant hydroxytyrosol was demonstrated to prevent high-fat diet-induced liver steatosis, by inhibiting oxidative stress, mitochondrial dysfunction and inflammation associated with steatosis (34,35). H2 was first reported to alleviate skin tumors by neutralizing toxic free radicals in 1975(37). In 2007, H2 was demonstrated to act as a therapeutic antioxidant by selectively reducing cytotoxic hydroxyl radical levels to improve focal I/R-induced brain injury in rats (38). Since then, H2 has been extensively investigated, where it has been shown to be an able antioxidant, anti-inflammatory and anti-apoptotic agent (10). The present study was undertaken to determine the effect of supplementation with exogenous H2 on acute alcohol-induced liver injury in mice.
Exogenous H2 can be supplied by 2% H2 gas inhalation (38), drinking H2-rich water (39), intraperitoneal injection of H2-rich saline (12) and intraperitoneal injection of H2 gas (19). Previous studies found that intraperitoneal injection of H2 gas can alleviate vascular remodeling (23), cardiac hypertrophy and dysfunction (20,21), and display neuroprotective effects in rabbits experiencing cardiac arrest (19). In the present study, it was observed that ethanol consumption induced hepatocyte injury as indicated by the upregulation of serum ALT and AST levels. Intraperitoneal injection with H2 gas was found to effectively protect against acute alcohol-induced liver injury and reduced serum TC levels. However, body weight was not influenced by the acute intraperitoneal injection of H2 gas or acute alcohol feeding. The lack of food intake records, liver weight, liver histological analysis and steatosis score and hepatic TG analysis are limitations of the present study. It has been documented that chronic alcohol over consumption may lead to hepatic steatosis, fibrosis and cirrhosis and eventually HCC (2,5,6). The long-term effects of intraperitoneal H2 injection on pathological features, such as hepatic fibrosis, and the effects of other H2 delivery methods, such as drinking H2-rich alcohol, on ALD, require further study.
Acute alcohol feeding activates cytochrome P450 2E1 and causes oxidative stress to activate JNK in hepatocytes, and JNK, in turn, reciprocally increases oxidative stress (33). JNK activation can cause programmed cell death, and increases the expression of lipogenic transcription factor sterol regulatory element binding protein (SREBP)-activated lipid synthesis enzymes, resulting in hepatic steatosis (31,33,40,41). Additionally, JNK may suppress hepatic peroxisome proliferator-activated receptor-α (PPAR-α) activation, which is a transcription factor and a positive regulator of intracellular free fatty acid and TG metabolism by regulating gene transcription involved in fatty acid transport and degradation in mitochondria and peroxisomes (32). Therefore, hepatic JNK activation increases oxidative stress, leads to hepatocyte apoptosis and injury, and contributes to hepatic steatosis via modulating lipid metabolic transcription factor activation. H2 has been shown to inhibit JNK activation in numerous liver disease and cardiovascular disease animal models (11,20,22,23). JNK inhibitor can improve acute alcohol-induced fatty liver and oxidative stress in mice (33). In the present study, phosphorylation of JNK in the liver induced by acute alcohol consumption was inhibited by the intraperitoneal injection of H2. Therefore, the protective effect of H2 against acute alcohol-induced liver injury is hypothesized to be partially mediated by reducing JNK phosphorylation. In addition to the inhibition of JNK activation, H2 has been shown to attenuate the activation of NF-κB in an LPS-induced cardiac dysfunction mice model (20). H2 has also been shown to increase the hepatic expression of nuclear factor erythroid 2-related factor 2 (Nrf2) (42) and PPAR-α (43) and reduced the hepatic expression of SREBP-1c (44) in NAFLD animal models. These transcription factors are essential mediators of inflammation (NF-κB), oxidative stress (Nrf2) and lipid metabolism (SREBP-1c and PPAR-α), where they have been reported to serve key roles in the pathogenesis of both ALD and NAFLD (35). Therefore, strategies modulating the expression or activation of these transcription factors may alleviate ALD and NAFLD (35,45-51). However, whether the protective effects of intraperitoneal injection of H2 against acute alcohol-induced liver injury is mediated by regulating the expression and/or activation of these transcription factors aforementioned requires further investigation.
The gut microbiome serves an important role in liver homeostasis, intestinal dysbiosis, including quantitative (such as intestinal bacterial overgrowth) and qualitative (such as colonic Firmicutes and Bacteroidetes levels) changes in the gut microbiota, and pathological bacterial translocation are fundamental for the pathogenesis of ALD (8,52). A number of these intestinal microbiota are affected by alcohol, which express hydrogenases and act as the main producers of endogenous H2 in humans and animals (10,20,52,53). Endogenous H2 is crucial for hepatic redox homeostasis, glucose and lipid homeostasis, in addition to immune and inflammatory homeostasis (11,54-57). Therefore, it remains of importance to investigate the effects of endogenous H2 in the pathogenesis of ALD.
In summary, findings of the present study indicated that intraperitoneal injection with exogenous H2 attenuated acute alcohol-induced liver injury in mice by inhibiting hepatic JNK activation. Therefore, it would be possible to treat alcohol-induced liver injury with H2, such as drinking H2-rich alcohol or H2-rich water, where H2 can be a potentially useful natural agent for the treatment of ALD.
Funding Statement
Funding: The present study was supported by National Natural Science Foundation of China (grant no. 81900376), Natural Science Foundation of Guangdong Province (grant no. 2018A030313657), the Project funded by China Postdoctoral Science Foundation (grant no. 2019M653238) and Guangdong famous Traditional Chinese Medicine inheritance studio construction project (grant no. 20180137).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
YZ, WG, MD and HY conceived and designed the experiments. YZ, GZ, ZC, MB and YH performed the experiments and analyzed the data. YZ drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University (IACUC code no: 2018-057).
References
The Original Article:
original title: Hydrogen gas alleviates acute alcohol-induced liver injury by inhibiting JNK activation
Yaxing Zhang, Mingmin Bi, Zifeng Chen, Min Dai, Ge Zhou, Yuxuan Hu, Hongzhi Yang, Weibing Guan
-
Abstract:
Binge alcohol drinking is fast becoming a global health concern, with the liver among the first organ involved and the one afflicted with the greatest degree of injury. Oxidative stress, alterations in hepatic metabolism, immunity and inflammation have all been reported to contribute to the development of alcoholic liver disease (ALD). Hydrogen gas (H2) serves a key role in the modulation of hepatic redox, immune and inflammatory homeostasis. However, the effects of treatment using intraperitoneal injection of H2 on ALD remain unexplored. Therefore, the aim of the present study was to investigate the effects and underlying mechanism of intraperitoneal injection of H2 on acute alcohol-induced liver injury in a mouse model. H2 was administered by daily intraperitoneal injections (1.0 ml/100 g) for 4 days. On day 4, the mice received H2 after fasting for 5.5 h. After 30 min, the mice were administered with 33% (v/v) ethanol at a cumulative dose of 4.5 g/kg body weight by four equally divided gavages at 20-min intervals. Blood and liver tissues were collected at 16 h after the first ethanol gavage. Subsequently, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride and total cholesterol (TC) levels were analyzed using an Automatic Clinical Analyzer. Hepatic JNK activity and GAPDH levels were examined by western blotting. It was observed that acute ethanol gavage induced liver injury, as indicated by significantly increased serum ALT and AST levels, which were effectively decreased by H2 at 16 h after the first ethanol gavage. In addition, H2 treatment reduced serum TC levels in the Alcohol+H2 group when compared with those in Alcohol group. Mechanistically, H2 attenuated hepatic JNK phosphorylation induced by acute ethanol gavage. Therefore, the results of the present study demonstrated that treatment with exogenous H2 by intraperitoneal injection may alleviate acute alcohol-induced liver injury by inhibiting hepatic JNK activation, which may represent a novel therapeutic strategy for ALD.