H2O2 promotes ghrelin secretion for neuroprotectionScientific Research

Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice
Akio Matsumoto
1Department of Pharmacology, Graduate School of Medicine, Chiba University, Chiba
2Division of Molecular Design, Medical Institute of Bioregulation, Kyushu University, Fukuoka
5Research Center for Nucleotide Pool, Kyushu University, Fukuoka, Japan
6These authors contributed equally to this work.
Megumi Yamafuji
3Laboratory of Pathophysiology, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka
6These authors contributed equally to this work.
Tomoko Tachibana
1Department of Pharmacology, Graduate School of Medicine, Chiba University, Chiba
Yusaku Nakabeppu
4Division of Neurofunctional Genomics, Medical Institute of Bioregulation, Kyushu University, Fukuoka
5Research Center for Nucleotide Pool, Kyushu University, Fukuoka, Japan
Mami Noda
3Laboratory of Pathophysiology, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka
5Research Center for Nucleotide Pool, Kyushu University, Fukuoka, Japan
Haruaki Nakaya
1Department of Pharmacology, Graduate School of Medicine, Chiba University, Chiba
Abstract
The therapeutic potential of molecular hydrogen (H2) is emerging in a number of human diseases and in their animal models, including in particular Parkinson’s disease (PD). H2 supplementation of drinking water has been shown to exert disease-modifying effects in PD patients and neuroprotective effects in experimental PD model mice. However, H2 supplementation does not result in detectable changes in striatal H2 levels, indicating an indirect effect. Here we show that H2 supplementation increases gastric expression of mRNA encoding ghrelin, a growth hormone secretagogue, and ghrelin secretion, which are antagonized by the β1-adrenoceptor blocker, atenolol. Strikingly, the neuroprotective effect of H2 water was abolished by either administration of the ghrelin receptor-antagonist, D-Lys3 GHRP-6, or atenolol. Thus, the neuroprotective effect of H2 in PD is mediated by enhanced production of ghrelin. Our findings point to potential, novel strategies for ameliorating pathophysiology in which a protective effect of H2 supplementation has been demonstrated.
Therapeutic applications of molecular hydrogen (H2) have been reported in a variety of human diseases and their animal models1, including ischemia-reperfusion injury2,3,4, metabolic syndrome5, diabetes mellitus type 26, organ transplantation7,8,9, reduction of adverse effects of anti-tumor drug therapy10,11 and radiation therapy12,13. Although the mechanism of action of H2 has not been clearly demonstrated, it is assumed that its anti-oxidative properties, particularly against hydroxyl radical (·OH) and peroxinitrite (ONOO−), are likely to underlie therapeutic efficacy2. Unlike other medical-gas therapy, H2 can be applied in air for inhalation or in solution for drinking, intravenous injection or dialysis. Whereas intravenous injection or dialysis delivers H2 directly into the blood stream, oral hydrogen-supplemented water (hydrogen water, H2-water) must be absorbed into the circulation resulting in limited H2 concentrations in the blood and in target organs7,14.
Parkinson’s disease (PD) has been a major focus in the field of oxidative stress and disease, because it is thought that degeneration of dopaminergic neurons can be triggered and aggravated by the accumulation of oxidative damage. However, although antioxidant therapies have been assessed in PD patients, clinical efficacy has not been established15,16. In contrast, a pilot study of hydrogen water therapy in PD patients has shown promising results17, and it has been reported that hydrogen water exhibits neuroprotective effects14 in the murine MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced PD model18. H2 levels were below measurable thresholds in the substantia nigra in PD model mice14, and hydrogen water, but not continuous inhalation of 2% H2, prevented the development of PD in a rat model19. These findings suggest that the therapeutic effects of hydrogen water may not require its anti-oxidant activity in the brain, and further that its efficacy requires processing that is consequent upon oral administration.
The purpose of the present study was to employ PD model mice to elucidate the underlying mechanism of the neuroprotective effects of oral H2-water. In particular, we hypothesized that oral H2 induces a messenger molecule, which travels to the brain and exerts neuroprotective activity.
Results
Oral hydrogen water induces ghrelin gene expression in the stomach
The stomach functions as an endocrine organ that secretes various peptide hormones with a broad range of physiological effects. We first focused on the stomach to investigate possible effects of oral H2-water at the level of gene induction. In a previous study14, it was reported that drinking H2-water for a period of 7 days prior to MPTP injection protected against MPTP toxicity. We administered oral H2-water for 4 consecutive days and analyzed expression in stomach tissue of gastrin, somatostatin, and ghrelin by real-time PCR method. Levels of ghrelin mRNA increased 1.9-fold in H2-water-treated versus control mice (Figure 1), whereas no effects of hydrogen water were detected on expression of the somatostatin gene (expressed only at trace levels) or the gastrin gene (expression of which was highly variable from individual to individual) (not shown).
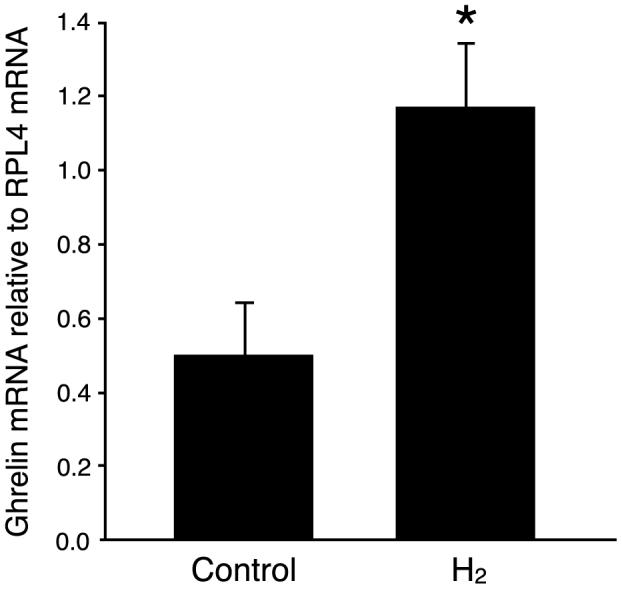
Control water, or H2 water (0.8 ml/mouse) made with a stick of magnesium (about 0.04 mM H2
To examine the time-course of ghrelin induction by hydrogen water, mice were administered hydrogen water or control water once a day for 0 (control) 1, 2 or 4 days and ghrelin levels were measured by ELISA in plasma derived from blood obtained 4–5 hours after the final administration of H2– or control water. Only mice that received H2 water for 4-day exhibited a significant increase in plasma ghrelin level, although mice administered hydrogen water for 2-day showed a non-significant increase (Figure 2).
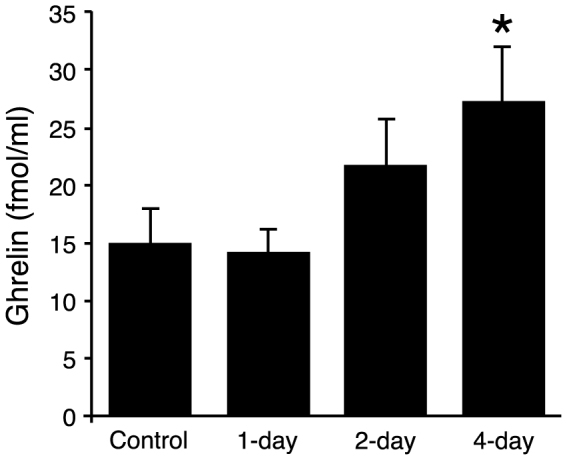
Mice (16–21 weeks, average 18.5 weeks, n = 5–10) received H2 water (or control water) as in Fig. 1 for 1, 2 or 4 d. Blood was collected on the final day of the experiment, 4 hr after the last ingestion, and plasma levels of ghrelin were quantified by ELISA. Panel shows data from male mice. The ghrelin level was significantly higher only in the group of mice drinking H2 water for 4 d. Data are represented as mean ± SEM.
β1-adrenergic receptor signaling mediates enhancement of ghrelin secretion by oral hydrogen water
It has been shown that gastric secretion of ghrelin is regulated by local environmental cues including blood glucose, estrogen, insulin and catecholamines20,21,22. In particular, it has been reported that β1-adrenergic receptor stimulation increases ghrelin secretion in vitro and in vivo22,23. We verified the expression of β1– and β2– adrenergic receptors in stomach tissue samples by real-time PCR method, and determined that there were no significant changes in the levels of expression after administration of oral H2-water for 4 days (data not shown). The increase in plasma ghrelin levels by oral hydrogen water was eliminated by administration of the β1-adrenergic receptor-specific blocker, atenolol (10 mg/kg i.p.) injected 30 min prior to H2 water administration on each of four days (Figure 3). Thus, activation of β1-adrenergic receptors is required for hydrogen water-induced enhancement of circulating ghrelin.
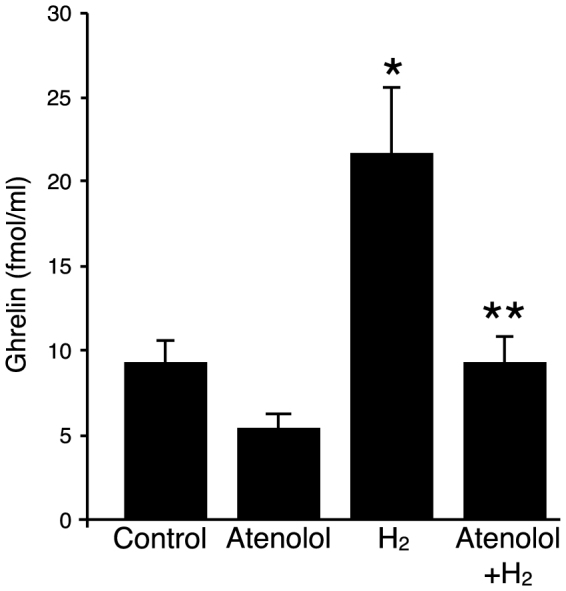
Plasma ghrelin levels were measured on the last day of experiment. Each group of mice (11–13 weeks of age; n = 4–5 for each group) was administered control water or H2 water (made with an open-air water electrolysis system) once a day for 4 d with or without i.p. injection of atenolol (10 mg/kg) prior to water ingestion. The H2 water group showed a significant increase in plasma ghrelin level compared to the control group (* p = 0.038), which was abrogated by pretreatment with atenolol (** p = 0.039). Datafare represented as mean ± SEM.
Blockades of ghrelin action abrogates the protective effect of H2 water in PD model mice
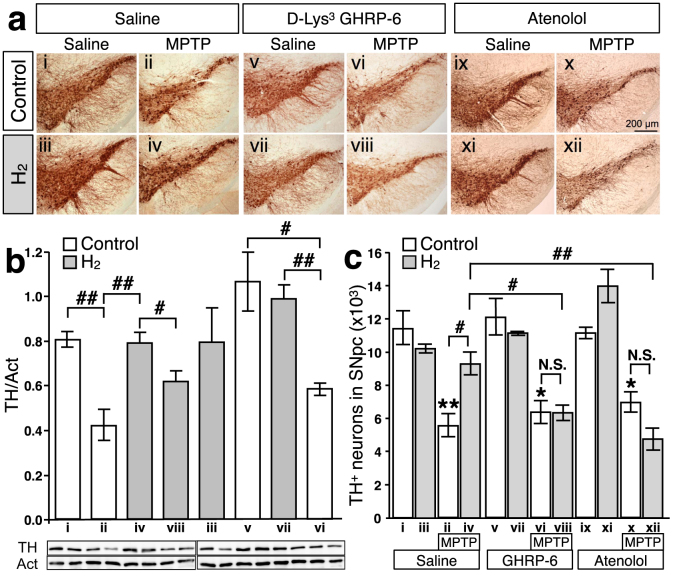
In a previous report, oral H2-water exhibited significant protective effects in MPTP-induced PD model mice against the loss of dopaminergic neurons from the substantia nigra14. To test directly the role of ghrelin, either the growth hormone secretagogue receptor antagonist, D-Lys3 GHRP-6 (100 nmol/day i.p.), or β1-adrenoceptor blocker, atenolol (10 mg/kg i.p.), was administered along with control or hydrogen water for 7 days prior to administration of MPTP. Loss of dopaminergic neurons from the substantia nigra was evaluated seven days following administration of MPTP. As shown in Figure 4, systemic administration of MPTP caused a significant loss of dopaminergic neurons from the pars compacta of the substantia nigra (SNpc) as assessed by immunohistochemical detection of tyrosine hydroxylase (TH) (a), and further confirmed by immunological detection of TH protein in the substantia nigra with actin as control (b), and stereological analysis (c). Whereas administration of H2 water alone in control mice had no effect, the loss of dopaminergic neurons in MPTP-treated mice was significantly decreased by administration of hydrogen water, as previously reported14. Strikingly, although administration of D-Lys3 GHRP-6 or atenolol in control mice had no effect alone and D-Lys3 GHRP-6 or atenolol did not affect MPTP-induced loss of dopaminergic neurons, the protective effects of hydrogen water were eliminated by either one of D-Lys3 GHRP-6 or atenolol. Thus, induction of gastric ghrelin production and subsequent activation of ghrelin-initiated signal transduction underlies the protective effects of hydrogen water in the MPTP model of PD.
Saturated H2 water was made daily as described in materials and methods. (a): Tyrosine hydroxylase (TH) staining was performed in substantia nigra pars compacta from saline-injected (i–iv), D-Lys3 GHRP-6-injected (v–viii), and atenolol-injected (ix–xii) mice, supplied with either normal tap water (control) or H2 water for 7 days. MPTP was injected (i.p.) after 7 days of D-Lys3 GHRP-6, atenolol, or saline-injection (ii, iv, vi, viii, x, and xii). (b): Summary of the immunoblotting analysis of TH protein in the substantia nigra tissue (n = 3–6 for each group) and the representative blot images were shown underneath (two samples per group). The TH band intensity was normalized to the band of actin on the same sample. (## p = 0.007, i vs. ii; 0.010, ii vs. iv; 0.004, vi vs. vii; # p = 0.043, iv vs. viii; 0.023, v vs. vi). Data in figures are represented as mean ± SEM. (c): Summary of the stereological analysis of nigral dopaminergic neurons. Although MPTP injections caused significant loss of TH-positive neurons (ii) (** p = 0.0001, i vs. ii), drinking H2 water for 7 days prior to MPTP-treatment significantly attenuates the loss of TH-positive cells (iv) (# p = 0.008, ii vs. iv). D-Lys3 GHRP-6, growth hormone secretagogue receptor antagonist, or β1-adrenoceptor blocker, atenolol, canceled the preservation effect of oral H2 water (viii and xii) (# p = 0.008; ii vs. iv; p = 0.003, iv vs. viii; p = 0.0003, iv vs. xii). Data in figures are represented as mean ± SEM. The statistical significance of data was assessed by one-way ANOVA followed by Benferroni test.
Discussion
Our findings demonstrate that the neuroprotective effects of oral hydrogen water, which produces negligible levels of H2 in the brain, result from gastric induction of the neuroprotective peptide hormone ghrelin and the subsequent activation of ghrelin receptors. In addition, we have shown an obligate role for β1-adrenergic receptors in hydrogen water-induced ghrelin up-regulation in plasma, consistent with previous reports that adrenergic stimulation regulates ghrelin release in vitro and in vivo22,24,25.
The neuroprotective effects of ghrelin in PD are well-established26, and it has been demonstrated that the receptor for ghrelin, the growth hormone secretagogue receptor (GHSR), is highly expressed by dopaminergic neurons of the substantia nigra27. It has been suggested that ghrelin protects nigrostriatal dopamine neurons via an uncoupling protein 2 (UCP2)-dependent mitochondrial mechanism28,29. However, we found that the expression of neither UCP2 mRNA nor protein was significantly upregulated by administration of H2 water drinking for 7 days (data not shown). This finding suggests an alternative signaling mechanism downstream of GHSR activation, perhaps involving PI3K/Akt30.
It was reported that administration of saturated hydrogen water (approx. 0.8 mM) led to symptomatic improvement in PD patients17. Administration of hydrogen water at about 0.05% saturation successfully maintained dopaminergic neurons in MPTP-induced PD model mice14. We employed three different methods to prepare hydrogen water (see Methods), which resulted in H2 concentration of 0.04–0.8 mM, and we observed that the effects of hydrogen water on ghrelin induction and protection of dopamine neurons were dose-independent over this range. Thus, small amounts of oral H2 are sufficient for gastric induction of ghrelin and subsequent neuroprotection, in the absence of detectable H2 in the brain. Interestingly, gut microbes can produce molecular hydrogen constitutively and lactulose, a synthetic disaccharide, is an effective substrate to enhance bacterial H2 production in the colon31. However, ingestion of lactulose had no significant effect on dopaminergic neuron survival in 6-OHDA-induced PD model rats (although alveolar H2 concentrations were elevated)19. These results emphasize the importance of gastric ghrelin induction in the neuroprotective action of H2. Oral H2-water is being explored as a therapeutic for PD as well as a variety of other human pathophysiological conditions17,32,33 under the generally held assumption that the mechanism of action of supplemental H2 is likely to reflect an antioxidative role. Our findings that oral H2 water exerts a neuroprotective effect through activation of an endogenous, gastric ghrelin system that is tightly coupled to β-adrenergic receptor signaling suggests the possibility of novel applications of H2 therapy for various diseases.
Methods
Animals
Procedures in animal experiments were approved by the university review board for animal care in Chiba University and Kyushu University, and performed in accordance with the guidelines established by the Science Council of Japan. All mice (C57BL/6J) were maintained in the animal facility under controlled temperature and lighting (12-hour light, 12-hour dark), and received a standard mouse chow diet and filter-sterilized tap water ad libitum.
Preparation and administration of H2– water
H2 water was prepared by one of three methods. The first method utilized the spontaneous ionization reaction of magnesium in water14. A magnesium stick (AZ31, Nakagawa Metal, Japan, composed of 96% magnesium, 3% aluminum, and 1% zinc) was polished to remove the oxidized surface and wiped clean with 1 N hydrogen chloride before dipping into drinking water for 15–20 min at 25°C. The second method was based on the electrolysis of water with platinum electrodes at 150 V DC for 20 min (open-air water electrolysis method). The anode was placed inside a drinking straw to vent generated oxygen. In the third method (employed in the experiments with MPTP-induced PD model mice), H2 gas was produced by solid polymer electrolyte water electrolysis method at 5 V DC in which the cathode and anode were separated by a solid polymer electrolyte membrane (Nafion® E.I., du Pont de Nemours & Co., Inc.) and collected through a polyethylene tube. Saturated H2 water was then generated by bubbling water in a drinking bottle with H2 gas such that H2 gas filled the headspace. H2 water made with this method and kept in sealed drinking bottles remained saturated with H2 for at least 24 hr.
In most experiments, each mouse received 0.8 ml of H2 water (administered within 30 min of preparation) or control water (obtained by boiling H2 water to degas) once every morning via feeding needle according to the experimental schedule. In experiments with MPTP-induced PD model mice, fresh H2 water was supplied every 24 hr and water intake was ad libitum for the 7 days prior to MPTP administration.
Measurements of ghrelin expression
Male and female mice aged between 41 and 48 weeks were chosen for the initial screening for the altered expression of gastric enzymes (n = 4 for each group), as they are relatively stable in dietary intake, which may influence mostly on the digestive enzyme expressions. Since the previous study was done with male mice14, the following experiments chose male mice. For the time-course analysis of ghrelin induction by hydrogen water, male mice (16–21 weeks, average 17.9 weeks, n = 5–10 in each group) were selected. Adrenoceptor mediated changes of ghrelin secretion was analyzed in male mice (age between 11–13 weeks, n = 4–5 for each group). Of note, there was no body weight change more than one gram during the period of experiment (4 days). These results are in line with the previous report with a recombinant ghrelin injection to mice, where the body weight change was below one gram in three days34.
Mice were administrated H2 water or control water for 4 days according to the experimental schedule. At the end of the experiment, mice were sacrificed by cervical dislocation and blood was collected by cardiopuncture with EDTA as anti-coagulant. Blood was centrifuged at 2000 g for 5 min at 4°C and the plasma was collected and mixed with 1 N hydrogen chloride (10% of plasma volume) to avoid inactivating deacylation of ghrelin20. Samples were stored at −80°C until analysis. Following blood collection, the stomach was removed intact and snap-frozen in liquid nitrogen for further analysis. For consistency, mice were killed 4–5 hours after the final administration of H2 water and at the same time of day. At the time of blood sampling the stomach was almost full of chow, which suggests that the water administration at a time (0.8 ml/time) through the feeding needle did not cause the sustained reduction on appetite.
The active form of ghrelin was measured in plasma by ELISA (Active Ghrelin ELISA kit, Sceti, Japan) according to the manufacturer’s instructions. For semi-quantitative PCR, stomach tissue frozen in liquid nitrogen was crushed into fine granules and total RNA was extracted with RNAiso (Takara, Japan) according to the manufacturer’s instructions. Two μg of total RNA was then used to synthesize first strand cDNA with a SuperScript VILO cDNA Synthesis Kit (Invitrogen, USA). mRNA expression levels were quantified by real-time PCR with SYBR green dye (Thunderbird Sybr qPCR Mix, Toyobo, Japan) with the specific primer sets shown in Table 1, and normalized to ribosomal protein L4 (RPL4) mRNA35,36,37.
Table 1
| RPL4.For | GCCAAGACTATGCGCAGGAAT |
|---|---|
| RPL4.Rev | GTAGCTGCTGCTTCCAGCTT |
| Ghrelin.For | TCAAGCTGTCAGGAGCTCAGTA |
| Ghrelin.Rev | TTGTCAGCTGGCGCCTCTT |
| Somatostatin.For | TGGCTGCGCTCTGCATCGT |
| Somatostatin.Rev | AGTACTTGGCCAGTTCCTGTT |
| Gastrin.For | CAGCGCCAGTTCAACAAGCT |
| Gastrin.Rev | ATTCGTGGCCTCTGCTTCTT |
| b1AR.For | CACTGTGGACAGCGATTCGA |
| b1AR.Rev | ACCTTGGACTCCGAGGAGAA |
| b2AR.For | ACAGGAACTGCTGTGTGAGGAT |
| b2AR.Rev | ACGCTAAGGCTAGGCACAGT |
Administration of ghrelin receptor antagonist, β1-adrenoceptor blocker, and MPTP
The ghrelin receptor antagonist, D-Lys3 GHRP-6 (Sigma-Aldrich, USA; 100 nmol/day), β1-adrenoceptor blocker, atenolol (ICI, USA; 10 mg/kg), or saline was administered by i.p. injection daily for 7 days in conjunction with supplying fresh control or H2 water. On day 7, MPTP-HCl (Sigma-Aldrich, USA; 15 mg/kg in 0.9% NaCl per injection) or saline as control was administrated by i.p. injection four times at 2 hr intervals. Mice were supplied with untreated tap water for the next 7 days before removal of brains under deep anesthesia (pentobarbital, 50 mg/kg i.p.).
Stereological and immunological analyses of nigral dopaminergic neurons
Stereological analysis was carried out as described14,38,39,40. In brief, coronal sections (30 μm thickness) were obtained through SNpc (−2.70 mm to −3.80 mm relative to bregma)41 with a MICROM cryostat. Free-floating sections were incubated with Block Ace (Dainippon Pharmaceutics, Japan) for 30 min followed by incubation with anti-tyrosine hydroxylase (TH) antibody (Chemicon, USA, 1:3000 in 10% Block Ace) for 2 days at 4°C. After rinsing, sections were immersed in a solution of 3% H2O2 in methanol/PBS (1:1) for 10 min at room temperature, followed by incubation for 2 hr in biotinylated goat anti-rabbit IgG (1:400, Vector, USA) and processing with a Vectastain ABC kit (Vector, USA) using 3′3′-diaminobenzidinetetrahydrochloride (DAB, Vector, USA) as peroxidase chromogen.
Unbiased stereological counts of TH-immunoreactive cell bodies in the SNpc were obtained using an optical fractionator method42 and Stereo Investigator software (Stereo Investigator 8, MicroBrightField Inc., USA). The boundary of SNpc was delineated under 100 × magnification and immunopositive neurons were counted in every third section (eight sections per brain) at 400 × magnification on a Nikon ECLIPSE 80 i microscope using a grid of 70 × 70 μm on a counting grid (75 × 100 × 12 μm) with 2 μm upper and lower guard zones. Gundersen’s coefficient of error in all samples was < 0.07.
Immunological detection and quantification of TH expression in the substantia nigra tissue were performed by western blotting with actin as control. Animals were prepared identically to those of the stereological analysis. The substantia nigra was removed and stored at −80°C until use. Samples were lysed on ice in hypotonic buffer (20 mM Hepes pH 7.6, 10 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 0.1% Triton X-100) with protease inhibitor cocktail (Roche, USA), and cleared by a centrifugation at 20 k G for 10 min at 4°C. Following quantification of protein concentration using the BCA method with BSA as control, five μg of tissue lysate reduced in sample buffer was resolved by 10% SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-TH antibody (1:2000) overnight at 4°C. Proteins were visualized using anti-rabbit secondary antibody conjugated to HRP and a chemiluminescence detection system (Immobilon Western Chemiluminescent HRP Substrate, Millipore, USA). Chemiluminescence image was quantified by Gauge application (Fuji Film, Japan) and the value was normalized to the level of actin band on the same sample specified by anti-Actin monoclonal antibody (Clone C4, Millipore, USA).
Statistical analysis and data managing
The statistical significance of data was assessed by unpaired Student t-test (two-tailed) unless otherwise mentioned and results were considered significant at p ≤ 0.05.
Author Contributions
A.M., Y.N., and M.N. conception and design of research; A.M., M.Y., T.T. performed experiments; A.M., M.Y., and M.N. analyzed data; A.M., M.Y., Y.N., and M.N. interpreted results of experiments; A.M. and M.Y. prepared figures; A.M., M.Y., Y.N., M.N., and H.N. wrote and revised manuscript; A.M., M.Y., T.T., Y.N., M.N., and H.N. approved final version of manuscript.
Acknowledgments
The authors are grateful to Dr. Douglas T. Hess (Case Western Reserve Univ.) for valuable advice and critical reading of the manuscript, and Dr. Yoshinori Tanaka (Corporate Engineering Division, Appliances Company, Panasonic Corporation, Japan) for help with hydrogen measurement. The authors also acknowledge the technical support of Mr. Yuichiro Kojima (Kyushu Univ.). This work was partly performed in the Cooperative Research Project Program of the Medical Institute of Bioregulation, Kyushu University. This work is supported in part by Grant-in-Aid for Scientific Research on Innovative Areas (MEXT 20117008 to A.M.), Grant-in-Aid for Exploratory Research (JSPS 24659111 to A.M.), Grant-in-Aid for Scientific Research (B) (JSPS 23390053 to H.N.), Grant-in-Aid for Scientific Research (S) (JSPS 22221004 to Y.N.), Grant-in-Aid for Scientific Research (C) (JSPS 22590084 to M.N.) and Academic Challenge in Robert T. Huang Entrepreneurship of Kyushu University (to M.Y.).
References
- Ohta S.
Recent Progress Toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Curr. Pharm. Des.
17, 2241–2252 (2011). [PMC free article] [PubMed] [Google Scholar] - Ohsawa I.
et al.
Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med.
13, 688–94 (2007). [PubMed] [Google Scholar] - Hayashida K.
et al.
H2 gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J. Am. Heart Assoc.
1, e003459 (2012). [PMC free article] [PubMed] [Google Scholar] - Fukuda K.
et al.
Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun.
361, 670–4 (2007). [PubMed] [Google Scholar] - Kamimura N., Nishimaki K., Ohsawa I. & Ohta S.
Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity (Silver Spring).
19, 1396–403 (2011). [PubMed] [Google Scholar] - Kajiyama S.
et al.
Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res.
28, 137–43 (2008). [PubMed] [Google Scholar] - Cardinal J. S.
et al.
Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int.
77, 101–109 (2009). [PubMed] [Google Scholar] - Kawamura T.
et al.
Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation
90, 1344–51 (2010). [PubMed] [Google Scholar] - Buchholz B. M.
et al.
Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant
8, 2015–24 (2008). [PubMed] [Google Scholar] - Nakashima-Kamimura N., Mori T., Ohsawa I., Asoh S. & Ohta S.
Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol.
64, 753–61 (2009). [PubMed] [Google Scholar] - Matsushita T., Kusakabe Y., Kitamura A., Okada S. & Murase K.
Investigation of protective effect of hydrogen-rich water against cisplatin-induced nephrotoxicity in rats using blood oxygenation level-dependent magnetic resonance imaging. Jpn. J. Radiol.
29, 503–12 (2011). [PubMed] [Google Scholar] - Qian L.
et al.
Radioprotective effect of hydrogen in cultured cells and mice. Free Radic. Res.
44, 275–82 (2010). [PubMed] [Google Scholar] - Schoenfeld M. P.
et al.
Hydrogen therapy may reduce the risks related to radiation-induced oxidative stress in space flight. Med. Hypotheses
76, 117–8 (2011). [PMC free article] [PubMed] [Google Scholar] - Fujita K.
et al.
Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS One
4, e7247 (2009). [PMC free article] [PubMed] [Google Scholar] - Jenner P.
Oxidative stress in Parkinson’s disease. Ann. Neurol.
53
Suppl 3S26–36; discussion S36–8 (2003). [PubMed] [Google Scholar] - Dexter D. T. & Jenner P.
Parkinson disease: from pathology to molecular disease mechanisms. Free Radic. Biol. Med.
62, 132–44 (2013). [PubMed] [Google Scholar] - Yoritaka A.
et al.
Pilot study of H2 therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov. Disord.
28, 836–9 (2013). [PubMed] [Google Scholar] - Heikkila R. E., Hess A. & Duvoisin R. C.
Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science
224, 1451–3 (1984). [PubMed] [Google Scholar] - Ito M.
et al.
Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med. Gas Res.
2, 15 (2012). [PMC free article] [PubMed] [Google Scholar] - Kojima M. & Kangawa K.
Ghrelin: structure and function. Physiol. Rev.
85, 495–522 (2005). [PubMed] [Google Scholar] - Kamegai J.
et al.
Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul. Pept.
119, 77–81 (2004). [PubMed] [Google Scholar] - Zhao T.
et al.
Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. U. S. A.
107, 15868–73 (2010). [PMC free article] [PubMed] [Google Scholar] - Gagnon J. & Anini Y.
Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology
153, 3646–56 (2012). [PubMed] [Google Scholar] - Gagnon J. & Anini Y.
Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology
154, 666–74 (2013). [PubMed] [Google Scholar] - Iwakura H.
et al.
Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3-1 in vitro. Endocrinology
152, 2619–25 (2011). [PubMed] [Google Scholar] - Andrews Z. B.
The extra-hypothalamic actions of ghrelin on neuronal function. Trends Neurosci.
34, 31–40 (2011). [PubMed] [Google Scholar] - Zigman J. M., Jones J. E., Lee C. E., Saper C. B. & Elmquist J. K.
Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol.
494, 528–48 (2006). [PMC free article] [PubMed] [Google Scholar] - Andrews Z. B.
et al.
UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature
454, 846–51 (2008). [PMC free article] [PubMed] [Google Scholar] - Andrews Z. B.
et al.
Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J. Neurosci.
29, 14057–65 (2009). [PMC free article] [PubMed] [Google Scholar] - Murata M.
et al.
Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J. Biol. Chem.
277, 5667–74 (2002). [PubMed] [Google Scholar] - Florent C.
et al.
Influence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study). J. Clin. Invest.
75, 608–13 (1985). [PMC free article] [PubMed] [Google Scholar] - Ishibashi T.
et al.
Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med. Gas Res.
2, 27 (2012). [PMC free article] [PubMed] [Google Scholar] - Kang K.-M.
et al.
Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med. Gas Res.
1, 11 (2011). [PMC free article] [PubMed] [Google Scholar] - Zhang W., Majumder A., Wu X. & Mulholland M. W.
Regulation of food intake and body weight by recombinant proghrelin. Am. J. Physiol. Endocrinol. Metab.
297, E1269–75 (2009). [PMC free article] [PubMed] [Google Scholar] - Nygard A., Jørgensen C. B., Cirera S. & Fredholm M.
Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol.
8, 67 (2007). [PMC free article] [PubMed] [Google Scholar] - Steinau M., Rajeevan M. S. & Unger E. R.
DNA and RNA references for qRT-PCR assays in exfoliated cervical cells. J. Mol. Diagn.
8, 113–8 (2006). [PMC free article] [PubMed] [Google Scholar] - Gao C.
et al.
Sensitivity of heterozygous α1,6-fucosyltransferase knock-out mice to cigarette smoke-induced emphysema: implication of aberrant transforming growth factor-β signaling and matrix metalloproteinase gene expression. J. Biol. Chem.
287, 16699–708 (2012). [PMC free article] [PubMed] [Google Scholar] - Kajitani K.
et al.
Galectin-1 promotes basal and kainate-induced proliferation of neural progenitors in the dentate gyrus of adult mouse hippocampus. Cell Death Differ.
16, 417–27 (2009). [PubMed] [Google Scholar] - Mallajosyula J. K.
et al.
Correction: MAO-B Elevation in Mouse Brain Astrocytes Results in Parkinson’s Pathology. PLoS One
7, 16 (2012). [PMC free article] [PubMed] [Google Scholar] - Mount M. P.
et al.
Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J. Neurosci.
27, 3328–37 (2007). [PMC free article] [PubMed] [Google Scholar] - Paxinos G. & Franklin K. B. J.
The mouse brain in stereotaxic coordinates.
2nd, (Academic Press, 2001). [Google Scholar] - West M. J., Slomianka L. & Gundersen H. J.
Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec.
231, 482–97 (1991). [PubMed] [Google Scholar]
The Original Article:
original title: Oral 'hydrogen water' induces neuroprotective ghrelin secretion in mice
Akio Matsumoto, Megumi Yamafuji, Tomoko Tachibana, Yusaku Nakabeppu, Mami Noda, Harumi Nakaya
-
Abstract:
The therapeutic potential of molecular hydrogen (H₂) is emerging in a number of human diseases and in their animal models, including in particular Parkinson’s disease (PD). H₂ supplementation of drinking water has been shown to exert disease-modifying effects in PD patients and neuroprotective effects in experimental PD model mice. However, H₂ supplementation does not result in detectable changes in striatal H₂ levels, indicating an indirect effect. Here we show that H₂ supplementation increases gastric expression of mRNA encoding ghrelin, a growth hormone secretagogue, and ghrelin secretion, which are antagonized by the β1-adrenoceptor blocker, atenolol. Strikingly, the neuroprotective effect of H₂ water was abolished by either administration of the ghrelin receptor-antagonist, D-Lys(3) GHRP-6, or atenolol. Thus, the neuroprotective effect of H₂ in PD is mediated by enhanced production of ghrelin. Our findings point to potential, novel strategies for ameliorating pathophysiology in which a protective effect of H₂ supplementation has been demonstrated.