Hydrogen water enhances Nrf2 expression to alleviate lung fibroblast injuryScientific Research

氢气水通过升高Nrf2的表达减轻百草枯诱导的肺纤维化
Hydrogen water alleviates paraquat-induced lung fibroblast injury in vitro by enhancing Nrf2 expression
李 婷
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
邓 树豪
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
雷 雯
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
李 振坤
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
吴 文娟
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
张 涛
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
董 昭兴
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
昆明医科大学第二附属医院呼吸与危重症科一病区,云南 昆明 650000,
First Ward of Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Kunming Medical University, Kunming 650000, China
Abstract
目的
探讨氢气水依赖Nrf2对百草枯诱导的肺成纤维细胞增殖,转化及胶原分泌的影响。
方法
人肺成纤维细胞(HFL-1)体外培养,采用RNA干扰技术沉默HLC-1细胞中Nrf2的表达,设置对照组、百草枯(PQ)染毒组、PQ+氢气水治疗组、PQ+氢气水+Nrf2siRNA综合干预组。设置对照组:人肺成纤维细胞(HFL1)正常培养;染毒组:HFL1+600 μmol/L PQ 24 h;氢气水治疗组:HFL1+600 μmol/L PQ24 h后+氢气水;综合干预治疗组:HFL1转染siRNA+600 μmol/LPQ 24 h后+氢气水。MTT法检测细胞的增殖,Western blot、qt-PCR、免疫荧光检测各组肺成纤维细胞Col-Ⅰ、Col-Ⅲ、α-SMA、Nrf2的表达,同时ELISA检测抗氧化物SOD、CAT、GSH的含量。
结果
氢气水治疗组较PQ染毒组Col-Ⅰ、Col-Ⅲ、α-SMA的表达明显降低,干扰Nrf2基因表达后,氢气水疗效较治疗组显著下降(P < 0.05)。氢气水治疗组Nrf2表达量较PQ染毒组增加(P < 0.05)。
结论
氢气水可能通过诱导Nrf2的表达促进肺成纤维细胞的增殖和抗氧化物的生成,同时抑制其转化和分泌功能。
Abstract
Objective
To investigate the effects of hydrogen water on proliferation, differentiation, collagen secretion and Nrf2 expression in paraquat-induced human lung fibroblasts.
Methods
In vitro cultured human lung fibroblasts (HFL1) exposed to 600 μmol/L paraquat (PQ) for 24 h were treated with hydrogen water with or without RNA interference of Nrf2 expression. The changes in the cell proliferation were examined using MTT assay, and the expressions of Col-I, Col-III, α-SMA and Nrf2 in the cells were detected using Western blotting, real-time quantitative PCR and immunofluorescence assay. The contents of SOD, CAT and GSH in the cells were determined with ELISA.
Results
Compared with the PQ-exposed cells, the cells with hydrogen water treatment showed significantly lowered expressions of Col-I, Col-III, and α-SMA. Interference of Nrf2 expression obviously attenuated the effect of hydrogen water on PQ-exposed cells. Hydrogen water treatment significantly increased the expression of Nrf2 and promoted the production of the antioxidants in PQ-exposed lung fibroblasts.
Conclusion
Hydrogen water enhances Nrf2 expression to promote the proliferation and production of antioxidants and inhibit the differentiation and collagen secretion in PQ-exposed human lung fibroblasts in vitro.
肺纤维化是一种由各种因素引起肺泡持续性损伤、细胞外基质过度沉积、成纤维细胞对肺部受损调节不受控制,从而导致肺部纤维化、正常肺组织结构改变以及功能丧失的疾病[1–3]。尽管近年来我们对IPF发病机理的认识和治疗有了一定的进展,但IPF患者的预后状况依然不容乐观。因此,为肺纤维化的治疗寻找新的切入靶点十分重要。氢气作为治疗性的医用气体,已经在多种疾病的实验和临床中被加以证实,但到目前为止,关于氢气的具体效应机制并不清楚[4–5]。有研究观察了氢气水减轻了百草枯(PQ)诱导的急性肺损伤,但对其具体的机制并没有详细的解释[5]。PQ中毒是一个世界性难题, 中毒后的死亡率高达50%~80%,迄今为止尚无有效的解毒药[6]。研究发现氧化应激反应在PQ中毒中具有非常重要的作用,而Nrf2作为抗氧化损伤的重要调节因子,在提高细胞抗氧化、抗炎、抗凋亡等方面有着重要作用[7]。研究发现氧化应激反应在PQ中毒中具有非常重要的作用[8],而Nrf2作为抗氧化损伤的重要调节因子,在提高细胞抗氧化、抗炎、抗凋亡等方面有着重要作用[9–10],但在肺纤维化中的具体机制尚未阐明。氢气作为抗氧化应激治疗物质,是否通过调节Nrf2表达在肺纤维化中发挥作用尚不清楚。本实验旨在通过干扰Nrf2基因的表达,观察氢气水治疗PQ诱导的肺成纤维细胞损伤的疗效,以探讨氢气水抗氧化反应在肺纤维化中发挥作用的可能机制,为肺纤维化的治疗提供新的方向。
1. 材料和方法
1.1. 材料
人肺成纤维细胞(HFL-1),昆明动物研究所提供;百草枯(江苏南通正达保护有限公司);DMEM培养基、胎牛血清、0.25%胰酶(Gibco);青霉素、链霉素(碧云天);总RNA提取试剂盒、逆转录试剂盒、蛋白提取试剂盒、内参抗体β-actin、鼠/兔二抗及荧光二抗(万类生物科技有限公司);一抗Nrf2、Col-Ⅰ、Col-Ⅲ、α-SMA(Abcam);ELISA检测SOD、CAT和GSH试剂盒(南京建成生物工程研究所);PCR引物(上海生物工程技术有限公司);siRNA引物(吉玛制药有限公司),Nrf2siRNA正义链:5′-GUAAGAAGCCAGAUGUUAAdUdU-3’,反义链:5′-dUdUCAUUCUUCGGUCUACAATT-3’。
1.2. 方法
1.2.1. HFL-1细胞的培养及鉴定
HFL-1细胞在含10%胎牛血清的DMEM培养基中,37 ℃,5% CO2条件下培养,取对数生长期的细胞, 使用0.25%胰酶消化法1:2进行传代。
1.2.2. siRNA沉默HFL-1细胞中Nrf2的表达
设计并合成3条针对Nrf2基因的siRNA序列,通过Western blot,qRT-PCR检测RNAi沉默肺成纤维细胞中的Nrf2的表达,筛选沉默效果最好的RNA片段,构建质粒表达载体,Nrf2-siRNA表达质粒转染肺成纤维细胞。
1.2.3. 实验分组及样本提取
取第3代对数生长期细胞,随机分为D1组:人肺成纤维细胞(HFL1)正常培养;D2组:HFL1+600 μmol/L PQ 24 h;D3组:HFL1+600 mol/L PQ 24 h后+氢气水;D4组:HFL1转染siRNA+600 mol/L PQ 24 h后+氢气水。氢气水按2×105个/mL加800 μL用量干预48 h后,提取各组蛋白及RNA用于后续实验。
1.2.4. MTT法检测细胞增殖
取生长良好细胞4×103/孔接种于96孔板,37 ℃,5% CO2条件下培养24 h,更换新鲜培养基,设置调零孔、对照孔、分组样本孔每组3个复孔。按分组处理细胞后孵育24 h,每孔加入10 μL MTT(5 mg/mL)工作液,水平摇床低速混匀1 min后,37 ℃,5% CO2培养箱中孵育4 h。吸尽孔内培养液,每孔加入100 μL甲攒溶解液,混匀后培养箱中孵育4 h,酶标仪检测各孔在波长570 nm的吸光值。分别于0、4、8、12、16、20、24 h检测细胞增殖状况。
1.2.5. Western blot检测Col-Ⅰ、Col-Ⅲ、α-SMA、Nrf2蛋白的表达
根据各样本质量及体积加入相应的预冷细胞裂解液,置冰上5 min充分裂解,12 000 r/min,4 ℃低温冷冻离心机离心10 min,取上清。BSA法测定蛋白浓度,根据测定浓度以50 μg每孔计算上样体积。SDS-PAGE电泳后转膜,5%脱脂奶粉封闭1 h,孵育一抗,内参抗体4 ℃过夜,洗膜后孵育二抗1 h,取膜ECL发光液显影。ImageJ软件以β-actin内参比值作为半定量依据,测定Wb图像灰度值。
1.2.6. Rt-PCR检测Col-Ⅰ、Col-Ⅲ、α-SMA、Nrf2mRNA的表达
RNA提取试剂盒提取总RNA,琼脂糖凝胶电泳检测RNA质量,逆转录试剂盒制备扩增模板,样本接种于96孔板,每组3个复孔并设置阴性对照。反应程序为95 ℃变性10 min,95 ℃变性30 s、58 ℃退火30 s、72 ℃ 30 min,40个循环,72 ℃延伸10 min。检测各模板CT值。CT值为每个反应管内的荧光信号达到设定阈值时所经历的循环数目。△△CT=△CT(实验值)-△CT(空白值)。以Folds=2-△ △
CT公式计算样本中mRNA的表达量。所有实验重复3次,计算平均值为实验结果。引物如下:Nrf2(正义链:5′-TCTCCATATCC CATTCCC-3’,反义链:5′-AAGGTGCTGAGTTGTTT T-3’);Col-Ⅰ(正义链:5′-GCAAGAACAGCGTAGCC TACAT-3’,反义链:5′-GCAGCCATCCACAAGCGT-3’);Col-Ⅲ(正义链:5′-AGATGATGGTGCTGAGAAG-3’,反义链:5′-TGGAAAGAAGTCTGAGGAAGG-3’);α- SMA(正义链:5′-AGGTAACGAGTCAGAGCTTTGG C-3’,反义链:5′-CTCTCTGTCCACCTTCCAGCAG-3’)。
1.2.7. 免疫荧光检测Col-Ⅰ、Col-Ⅲ、α-SMA、Nrf2的表达
生长良好细胞3×105 /孔接种于铺好爬片的24孔板,37 ℃,5% CO2条件下培养24 h,更换新鲜培养基后按不同分组处理细胞,每组3个复孔,设置对照孔、调零孔,24 h后进行免疫荧光检测。吸尽培养基后PBS浸洗3次,用4%多聚甲醛固定爬片30 min,PBS浸洗3次后正常山羊血清室温封闭30 min,去除封闭液加一抗湿盒孵育过夜,PBST浸洗3次后荧光二抗避光孵育1 h,浸洗后DAPI染核10 min,PBST浸洗后封片看片,分析结果。
1.2.8. ELISA检测抗氧化物SOD、CAT、GSH蛋白的含量
取生长良好细胞蛋白裂解液提取蛋白,按SOD、CAT、GSH ELISA检测试剂盒说明书操作。
1.2.9. 统计学分析
本实验数据采用均数±标准差表示,使用SPSS18.0统计学软件进行单因素方差分析。使用t检验进行组间统计学比较,P < 0.05认为差异具有统计学意义。
2. 结果
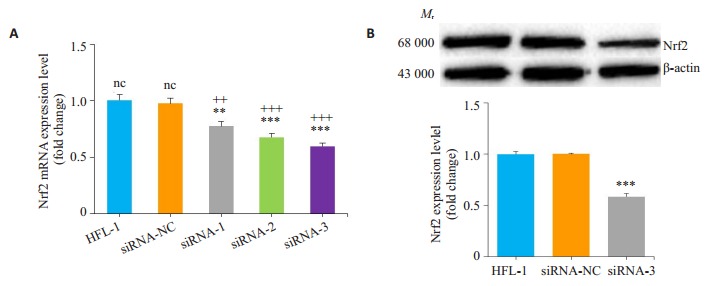
2.1. Nrf2-siRNA沉默HFL-1细胞中Nrf2的表达
设计并合成3条针对Nrf2基因的siRNA序列,设置空白对照组、siRNA对照组、siRNA- Nrf2(siRNA-Nrf2-1,siRNA-Nrf2-2,siRNA-Nrf2-3)组,Rt-PCR检测各组Nrf2结果显示,Nrf2表达量空白对照组最高,siRNA对照组与空白对照组表达量一致,siRNA-Nrf2组与空白对照组比较均降低,其中siRNA-Nrf2-3 Nrf2表达量最低,抑制效果最明显(图 1A)。筛选siRNA-Nrf2-3构建质粒表达载体转染肺成纤维细胞用于后续分组处理。Western blot检测各组Nrf2结果显示,Nrf2表达量空白对照组最高,siRNA对照组与空白对照组表达量一致,siRNA-Nrf2-3组表达量被明显抑制(图 1B)。
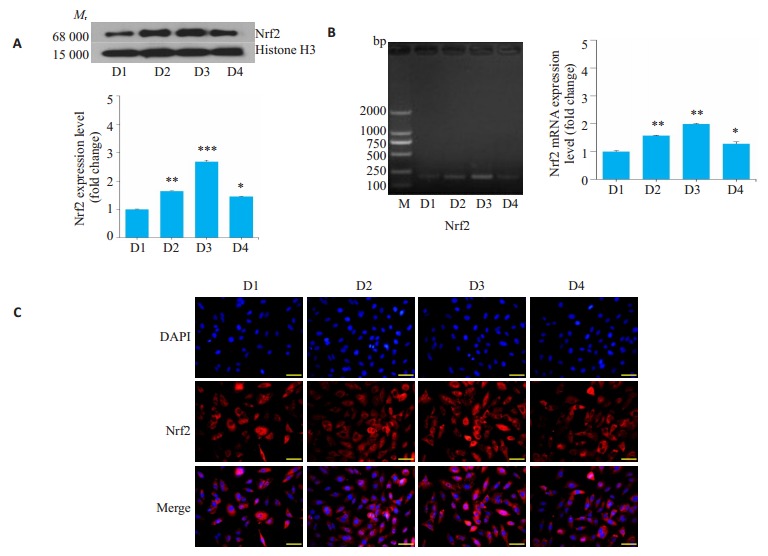
2.2. 氢气水增强PQ染毒肺成纤维细胞Nrf2的表达
Western blot检测各组Nrf2结果显示,空白对照组Nrf2蛋白表达量最低,PQ染毒组明显增加,而PQ 24 h+氢气水治疗组表达量最高,PQ 24 h+氢气水+siRNA综合干预组表达量高于空白对照组,但介于空白对照组和染毒组之间(图 2A)。Rt-PCR(图 2B),免疫荧光(图 2C)结果与Western blot结果一致。
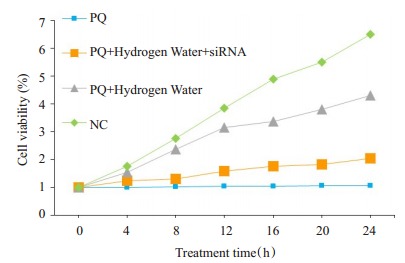
2.3. 氢气水调节Nrf2促进PQ染毒肺成纤维细胞的增殖作用
通过MTT试剂盒,检测不同处理的肺成纤维细胞增殖情况(图 3),设置重复样板,加氢气水后,每4 h检测一次,24 h动态分析结果显示,空白对照组细胞增长趋势良好,PQ染毒组细胞增殖明显受限;在PQ染毒24 h后给与氢气水治疗,24 h监测发现细胞增长趋势明显上升;当siRNA抑制Nrf2基因后,给与氢气水治疗PQ 24 h后的HFL-1细胞,细胞增长再次明显受到抑制,且介于PQ染毒组和氢气水治疗组之间。
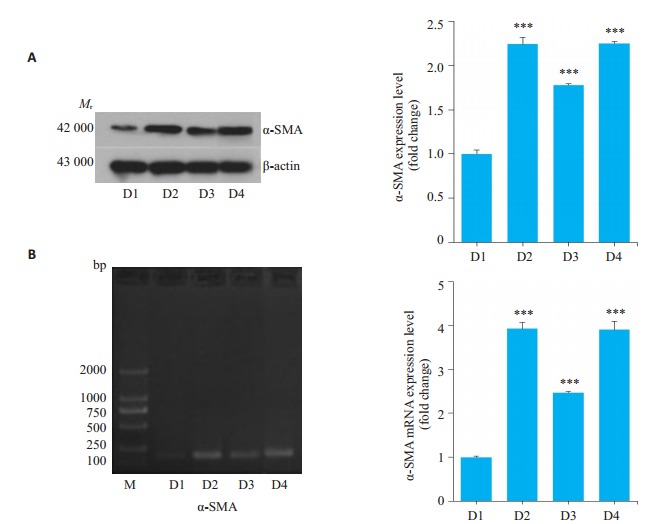
2.4. 氢气水调节Nrf2抑制PQ染毒肺成纤维细胞的转化作用
Western blot检测各组α-SMA结果显示,与空白对照组相比,PQ染毒组肌成纤维细胞标志物α-SMA蛋白表达量显著升高,给予氢气水治疗后其表达量显著降低,在siRNA沉默Nrf2基因后,其表达量又进一步上升,较PQ染毒组相差不明显(图 4A)。Rt-PCR结果与Western blot结果一致(图 4B)。
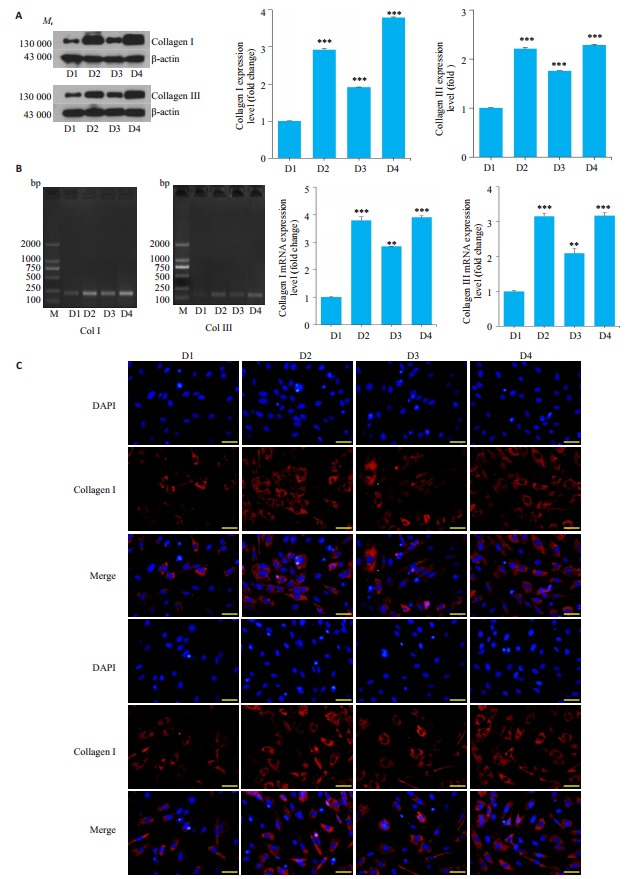
2.5. 氢气水调节Nrf2抑制PQ染毒肺成纤维细胞胶原分泌的影响
Western blot检测各组Col-Ⅰ、Col-Ⅲ结果显示,与空白对照组相比,PQ染毒组胶原蛋白的表达量显著升高,给予氢气水治疗后其表达量显著降低,在siRNA沉默Nrf2基因后,其表达量又进一步上升,较PQ染毒组相差不明显(图 5A)。Rt-PCR(图 5B),免疫荧光(图 5C)结果与western blot结果一致。
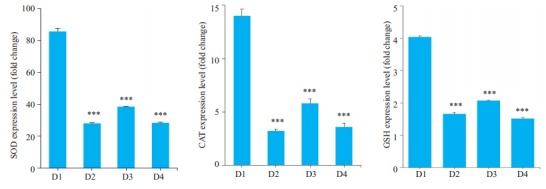
2.6. 不同处理组细胞抗氧化物SOD、CAT、GSH的含量
ELISA检测抗氧化物SOD、CAT、GSH的含量结果显示,空白对照组SOD、CAT、GSH蛋白表达量最高,PQ染毒组总体最低,而PQ+氢气水治疗组表达量增加,介于空白对照组与染毒组之间,PQ+氢气水+siRNA综合干预组表达量较PQ染毒组相差不明显(图 6)。
3. 讨论
PQ中毒常导致严重的肺损伤,缓解期肺部组织常发生纤维化病变,近年来,多数学者认为氧化应激反应是PQ中毒致肺损伤的重要因素之一[11–14],但其具体的致病机制尚不清楚。在肺纤维化的发展过程中,肺成纤维细胞大量向肌成纤维细胞转化,同时细胞外基质大量分泌[15–17]。因此,从氧化应激反应方向研究肺成纤维细胞在肺纤维化中具体的致病机制,对于寻找肺纤维化的有效治疗方法十分重要。本研究发现氢气水可以缓解PQ所致的肺成纤维细胞损伤,且这一作用很可能通对Nrf2细胞因子的调节发挥作用,得出氢气水通过Nrf2因子减轻百草枯诱导的肺纤维化的结论,为肺纤维化治疗提供了新的思路。
氢气水对细胞氧化应激损伤具有保护作用已被多篇研究报导,但氢气水具体通过何种机制发挥其抗氧化损伤的作用,目前尚无详细报道[18]。本研究通过使用氢气水干预百草枯染毒肺成纤维细胞,观察其增殖、转化、胶原分泌及抗氧化物质的变化。结果发现,氢气水干预后,染毒细胞的转化及胶原分泌减少,而细胞抗氧化物质表达量增多,且缓解了染毒细胞生长下降的趋势。氢气水通过增强细胞的抗氧化能力,减轻了百草枯对肺成纤维细胞损伤的作用。有研究[19]通过四氯化碳,硫代乙酰胺和胆管结扎治疗诱导肝纤维化小鼠模型后给予富氢水治疗,同时提取肝细胞肝星状细胞与氢培养,结果证实了富氢水通过清除羟自由基保护肝细胞免受损伤,从而抑制肝纤维化形成。Gao等[20]通过吸入氢气干预博来霉素诱导的小鼠肺纤维化,得出氢气通过抑制转化生长因子(TGF-β)及氧化应激相关信号通路的表达,减弱小鼠肺组织纤维化。此外, 相关研究还指出高浓度吸氧,内毒素诱导等造成的肺损伤,可以通过吸入氢气得以改善[21–23]。这提示,氢气水可以通过调节氧化应激缓解各种因素所致的肺损伤,可能是潜在的干预药物,但其具体的作用靶点还有待进一步研究。
Nrf2作为抗氧化反应元件调控的主要因子,其抗氧化和细胞保护基因在肺组织对抗氧化性疾病中具有重要的保护作用[24]。相关文献指出,Nrf2在类毒素(LPS)所致的急性肺损伤中具有保护作用[25],同时,H2被报道通过Nrf2/HMGB1途径抑制脓毒症小鼠肺损伤,指出Nrf2在H2治疗感染性肺损伤中起重要作用[26]。有研究发现,从Nrf2缺失小鼠体内提取的成纤维细胞与野生型小鼠的细胞相比,其抗氧化酶基因水平下降。经PQ处理两组细胞后,Nrf2基因缺失组细胞的活性氧(ROS)水平明显升高,细胞存活率降低,而野生小鼠组细胞Nrf2表达增加,同时抗氧化物质GSH浓度增高[27–28]。Nrf2的在肺纤维化组织中的上调,在肺纤维化的发生机制中对于调控机体氧化还原状态可能有着至关重要的作用被多篇文献报道[29–30]。本研究假设氢气水可能通过调控氧化应激通路的某个关键信号分子发挥减轻百草枯对肺成纤维细胞损伤的作用。通过抑制抗氧化损伤重要调节因子Nrf2的表达,观察氢气水对PQ染毒组细胞损伤的疗效,发现细胞增长较单独的PQ染毒组再次明显受到抑制,细胞胶原大量分泌,且抗氧化物质表达量也减少,氢气水缓解肺成纤维细胞损伤的疗效显著降低。这一研究结果提示,氢气水具有减轻百草枯对肺成纤维细胞损伤的作用,这一作用很可能通过对Nrf2细胞因子的调节发挥作用。进一步检测氢气水治疗组细胞Nrf2基因的表达发现,Nrf2基因表达显著增高,这可能是由于氢气水在发挥抗氧化损伤作用的过程中,诱导Nrf2细胞因子的分泌,并依赖Nrf2诱导抗氧化物质的分泌,从而发挥抗氧化应激保护细胞的作用。
综上所述,本研究发现氢气水能缓解PQ所致的肺成纤维细胞损伤,同时能够诱导肺成纤维细胞Nrf2的表达升高,当细胞中的Nrf2被抑制后, 其通过增强肺成纤维细胞细胞抗氧化能力从而减轻肺细胞损伤的疗效大大降低。本研究结果从肺成纤维细胞的角度,探讨了氢气水治疗PQ所致肺损伤的作用机制,得出氢气水通过依赖Nrf2因子减轻百草枯诱导的肺纤维化的结论,为肺纤维化治疗提供了新的思路。
Funding Statement
国家自然科学基金(81560015,81860018);云南省科技厅-昆明医科大学应用基础研究联合专项重点项目、面上项目(2017FE468-005,2017FE468-210,2014FB046);云南省教育厅研究生项目(K13219126);昆明医科大学研究生创新基金(2019S147)
Supported by National Natural Science Foundation of China (81560015, 81860018)
References
[Maher TM. Beyond the diagnosis of idiopathic pulmonary fibrosis; the growing role of systems biology and stratified medicine[J]. Curr Opin Pulm Med, 2013, 19(5): 460-5.] [PubMed] [CrossRef] [Google Scholar]
[Meyer KC. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis[J]. Expert Rev Respir Med, 2017, 11(5): 343-59.] [PubMed] [Google Scholar]
[Moodley Y, Ilancheran S, Samuel C, et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair[J]. Am J Respir Crit Care Med, 2010, 182(5): 643-51.] [PubMed] [CrossRef] [Google Scholar]
[Ohta S. Recent progress toward Hydrogen medicine: potential of molecular Hydrogen for preventive and therapeutic applications[J]. Curr Pharm Des, 2011, 17(22): 2241-52.] [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[Li L, Liu T, Liu L, et al. Effect of hydrogen-rich water on the Nrf2/ ARE signaling pathway in rats with myocardial ischemia-reperfusion injury[J]. J Bioenerg Biomembr, 2019, 51(6): 393-402.] [PubMed] [CrossRef] [Google Scholar]
[田华, 姚树桐, 高凤美.含氢水对实验性小鼠肺纤维化的抑制作用及机制[J].中国现代医学杂志, 2012, 22(2): 29-33.] [CrossRef] [Google Scholar]
[雷亚莉, 张新彧, 刘瑛.急性百草枯中毒肺损伤机制和临床治疗研究进展[J].陕西医学杂志, 2018, 47(11): 1510-3.] [CrossRef] [Google Scholar]
[Sun B, Chen YG. Advances in the mechanism of paraquat-induced pulmonary injury[J]. Eur Rev Med Pharmacol Sci, 2016, 20(8): 1597-602.] [PubMed] [Google Scholar]
[Todorovic M, Wood SA, Mellick GD. Nrf2: a modulator of Parkinson’s disease[J]? J Neural Transm, 2016, 123(6): 611-9.] [PubMed] [CrossRef] [Google Scholar]
[吕晓梅, 马丽杰.转录因子Nrf在肝脏疾病中的作用[J].生物化学与生物物理进展, 2015, 42(10): 920-5.] [Google Scholar]
[Dinis- Oliveira RJ, Duarte JA, Sánchez- Navarro A, et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment[J]. Crit Rev Toxicol, 2008, 38(1): 13-71.] [PubMed] [Google Scholar]
[彭志允, 常平, 王华, 等.高强度血液灌流联合长时程血液滤过在百草枯中毒救治中的应用:1例报告[J].南方医科大学学报, 2017, 35 (10): 1515-8.] [PubMed] [Google Scholar]
[Xu Y, Tai W, Qu X, et al. Rapamycin protects against paraquat-induced pulmonary fibrosis: Activation of Nrf2 signaling pathway [J]. Biochem Bioph Res Co, 2017, 490(2): 535-40.] [PubMed] [CrossRef] [Google Scholar]
[成子佳, 常云峰.百草枯中毒的机制及诊疗进展[J].医学综述, 2018, 24(20): 4033-8.] [CrossRef] [Google Scholar]
[穆颖, 许朝晖. MicroRNAs在肺癌发病和诊疗中作用的研究进展[J].分子影像学杂志, 2015, 38(2): 139-41.] [CrossRef] [Google Scholar]
[Vancheri C, Gili E, Failla M, et al. Bradykinin differentiates human lung fibroblasts to a myofibroblast phenotype via the B2 receptor [J]. J Allergy Clin Immunol, 2005, 116(6): 1242-8.] [PubMed] [CrossRef] [Google Scholar]
[赵运旺, 朱家宁.肺癌肿瘤标志物神经元特异性烯醇化酶抗原适配子的筛选及其应用[J].分子影像学杂志, 2017, 40(1): 112-5.] [CrossRef] [Google Scholar]
[Ohta S. Initiation, development and potential of Hydrogen medicine: Toward therapeutic and preventive applications of molecular Hydrogen against a variety of diseases[J]. Seikagaku of Japanese Biochemical Society, 2015, 87(1): 82-90.] [PubMed] [Google Scholar]
[Koyama Y, Taura K, Hatano E, et al. Effects of oral intake of Hydrogen water on liver fibrogenesis in mice[J]. Hepatol Res, 2014, 44(6): 663-77.] [PubMed] [CrossRef] [Google Scholar]
[Gao L, Jiang D, Geng J, et al. Hydrogen inhalation attenuated bleomycin- induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial- to-mesenchymal transition[J]. Exp Physiol, 2019, 104(12): 1942-51.] [PubMed] [CrossRef] [Google Scholar]
[Kawamura T, Wakabayashi N, Shigemura N, et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo[J]. Am J Physiol Lung Cell Mol Physiol, 2013, 304(10): L646-56.] [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[Papaiahgari S, Yerrapureddy A, Hassoun PM, et al. EGFR-activated signaling and actin remodeling regulate cyclic stretch-induced NRF2-ARE activation[J]. Am J Respir Cell Mol Biol, 2007, 36(3): 304-12.] [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[盛洁莹, 谢旺, 厉旭光, 等.氢气饱和生理盐水对内毒素诱导的小鼠急性肺损伤的保护作用[J].同济大学学报:医学版, 2017, 38(1): 36-9, 43.] [Google Scholar]
[Boutten A, Goven D, Artaud-Macari E, et al. Protective role of Nrf2 in the lungs against oxidative airway diseases[J]. Med Sci (Paris), 2011, 27(11): 966-72.] [PubMed] [CrossRef] [Google Scholar]
[Yu JB, Shi J, Gong LR, et al. Role of Nrf2/ARE pathway in protective effect of electroacupuncture against endotoxic shock-induced acute lung injury in rabbits[J]. PLoS One, 2014, 9(8): e104924.] [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[Yang M, Yu Y, Xie K. Effects of hydrogen on lung injury in wild-type and Nrf2 gene knockout mice: relationship with Nrf2/HO-1/ HMGB1 pathway[J]. Zhonghua wei chong bing ji jiu yi xue, 2019, 31(7): 862-6.] [PubMed] [Google Scholar]
[Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress[J]. Antioxid Redox Signal, 2006, 8(1/2): 76-87.] [PubMed] [Google Scholar]
[Cho HY, Reddy SP, Debiase A, et al. Gene expression profiling of NRF2- mediated protection against oxidative injury[J]. Free Radic Biol Med, 2005, 38(3): 325-43.] [PubMed] [Google Scholar]
[Shao X, Chen JH. Progress on pathogenesis and treatment of paraquat-induced pulmonary fibrosis[J]. Zhejiang Da Xue Xue Bao Yi Xue Ban, 2014, 43(6): 717-27.] [PubMed] [Google Scholar]
[Ruan YJ, Jian XD, Guo GR. Progress in research on mechanism of pathogenesis and treatment of acute paraquat poisoning [J]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 2009, 27(2): 114-6.] [PubMed] [Google Scholar]
The Original Article:
original title: Hydrogen water alleviates paraquat-induced lung fibroblast injury in vitro by enhancing Nrf2 expression
Ting Li, Shuhao Deng, Wei Lei, Zhengkun Li, Wenjuan Wu, Tao Zhang, Zhaoxing Dong
-
Abstract:
Objective: To investigate the effects of hydrogen water on proliferation, differentiation, collagen secretion and Nrf2 expression in paraquat-induced human lung fibroblasts.
Methods: In vitro cultured human lung fibroblasts (HFL1) exposed to 600 μmol/L paraquat (PQ) for 24 h were treated with hydrogen water with or without RNA interference of Nrf2 expression. The changes in the cell proliferation were examined using MTT assay, and the expressions of Col-I, Col-III, α-SMA and Nrf2 in the cells were detected using Western blotting, real-time quantitative PCR and immunofluorescence assay. The contents of SOD, CAT and GSH in the cells were determined with ELISA.
Results: Compared with the PQ-exposed cells, the cells with hydrogen water treatment showed significantly lowered expressions of Col-I, Col-III, and α-SMA. Interference of Nrf2 expression obviously attenuated the effect of hydrogen water on PQ-exposed cells. Hydrogen water treatment significantly increased the expression of Nrf2 and promoted the production of the antioxidants in PQ-exposed lung fibroblasts. Conclusions: Hydrogen water enhances Nrf2 expression to promote the proliferation and production of antioxidants and inhibit the differentiation and collagen secretion in PQ-exposed human lung fibroblasts in vitro.