H2 Supplementation Enhances Submaximal Exercise PerformanceScientific Research

Acute Supplementation with Molecular Hydrogen Benefits Submaximal Exercise Indices. Randomized, Double-Blinded, Placebo-Controlled Crossover Pilot Study
Tyler W. LeBaron
1Department of Kinesiology and Outdoor Recreation, Southern Utah University, UT,
USA
2Molecular Hydrogen Institute, UT,
USA
3Slovak Academy of Sciences, Centre of Experimental Medicine, Institute for Heart Research, Bratislava,
Slovakia
Abigail J. Larson
1Department of Kinesiology and Outdoor Recreation, Southern Utah University, UT,
USA
Shigeo Ohta
4Department of Neurology, Juntendo University School of Medicine, Tokyo,
Japan
Toshio Mikami
5Department of Sport Science, Nippon Medical School, Tokyo,
Japan
Jordon Barlow
1Department of Kinesiology and Outdoor Recreation, Southern Utah University, UT,
USA
Josh Bulloch
1Department of Kinesiology and Outdoor Recreation, Southern Utah University, UT,
USA
Mark DeBeliso
1Department of Kinesiology and Outdoor Recreation, Southern Utah University, UT,
USA
USA
USA
Slovakia
Japan
Japan
Abstract
Background
Clinical studies have reported hydrogen-rich water (HRW) to have therapeutic and ergogenic effects. The aim of this study was to determine the effect of acute supplementation with HRW on exercise performance as measured by VO2, respiratory exchange ratio (RER), heart rate (HR), and respiratory rate (RR).
Methods
Baseline levels of all exercise indices were determined in nineteen (4 female, 23.4 ± 9.1 yr; 15 male, 30.5 ± 6.8 yr) healthy subjects using a graded treadmill exercise test to exhaustion. Each subject was examined two additional times in a randomized double-blinded, placebo-controlled crossover fashion. Subjects received either HRW or placebo, which was consumed the day before and the day of the testing. HRW was delivered using the hydrogen-producing tablets, DrinkHRW (5 mg of H2). All data was analyzed with SPSS using pairwise comparisons with Bonferroni adjustment.
Results
HRW supplementation did not influence maximal or minimal indices of exercise performance (VO2, RER, HR and RR) (p < 0.05). However, HRW significantly decreased average exercising RR and HR (p < 0.05). HRW decreased exercising HR during minutes 1–9 of the graded exercise test (121 ± 26 bpm) compared to placebo (126 ± 26 bpm) and baseline (124 ± 27 bpm) (p < 0.001) without substantially influencing VO2.
Conclusion
Acute supplementation of DrinkHRW tablets may benefit submaximal aerobic exercise performance by lowering exercising HR. Further studies are needed to determine the influence and practical significance of HRW on varying exercise intensities as well as optimal dosing protocols and the effects of chronic use.
INTRODUCTION
Ergogenic supplements are popular among athletes who are hoping to enhance athletic performance, reduce fatigue, improve recovery, and prevent exercise-induced harm from reactive oxygen/nitrogen species (ROS/RNS) and excessive inflammation [1,2]. Antioxidants and anti-inflammatories are often consumed by athletes to prevent the harmful effects of ROS/RNS, and inflammation; however, many of the benefits of exercise may be mediated by the exercise-induced production of ROS and inflammation [3].
Some studies have demonstrated that supplementation with conventional antioxidants results in deleterious effects that can negate the benefits of exercise training (e.g., mitochondrial biogenesis) [4]. A likely explanation could be that exogenous antioxidants are not effective at maintaining redox homeostasis, but may instead exacerbate redox dysregulation [5].
Hydrogen gas (H2), which can easily permeate bio-membranes and subcellular compartments [6], may have ergogenic properties due to its anti-inflammatory and anti-oxidant-like effects [7]. Although H2 has antioxidant-like effects and is considered a reducing agent in organic chemistry, it is too stable to react with important signaling oxidants (e.g., •O2−, NO•, H2O2) [6,8]. This mitigates the risk of hampering exercise training benefits. Hydrogen appears to act via hormesis in which it activates the Nrf2 pathway and subsequent induction of phase 2 antioxidant enzymes [9]. Additionally, H2 modulates signal transduction, protein phosphorylation cascades, and gene expression. These influences can favorably modulate inflammatory pathways (NFAT, NF-κB, TLRs) [10–12], increase PGC-1α (marker of mitochondrial biogenesis) [13,14], maintain mitochondrial membrane potential and ATP production [14,15], increase resilience to stress [16], and influence other signaling molecules (e.g., Nrf2, Nox1,STAT3, etc.) [11].
Results from clinical studies suggest inhalation of hydrogen gas or ingestion of hydrogen-rich water (HRW) may benefit individuals who have experienced or are experiencing cerebral infarction [17], post-cardiac arrest syndrome [18], rheumatoid arthritis [19], mild cognitive impairments [20], Parkinson’s disease [21], metabolic syndrome [22,23], mitochondrial myopathies [24] and other diseases previously reviewed [7]. The effect of HRW on measurements of exercise performance, recovery, and tolerance to submaximal and maximal exercise in healthy and diseased populations is less well documented. However, these studies suggest that HRW may prevent early fatigue and reduce lactate levels [25,26], improve range of motion and recovery from soft tissue injury [27], attenuate delayed onset muscle soreness from eccentric exercise [28], prevent exercise-induced decline in antioxidant enzymes [28], and improve the ability to maintain peak power output during repetitive sprints [29]. Most of these athletic studies examined the effect of chronic supplementation (H2 administration for over 24 hours). Therefore, the purpose this double-blind repeated measures study was to investigate the effects of acute hydrogen supplementation, compared to placebo, on indices of submaximal and maximal aerobic exercise performance as measured by VO2, respiratory exchange ratio (RER), heart rate (HR), and respiratory rate (RR) in a young, non-diseased population.
MATERIALS AND METHODS
1. Participants
Twenty healthy subjects between the ages of 18–45 years (4 female, 23.4 ± 9.1 yr; 15 male, 30.5 ± 6.8 yr) were recruited to participate in a study to evaluate the effects of acute supplementation of H2-producing tablets (DrinkHRW) on exercise performance. After approval by the institutional review board at Southern Utah University, participants were recruited by word of mouth and provided informed consent and risk evaluation. Exclusion criteria included any condition that would impair maximal effort in a graded treadmill exercise test such as smoking, diabetes, obesity, pregnancy, post-menopausal, etc. Physical characteristics of the subjects are presented in Table 1. Each subject completed three maximal graded exercise treadmill tests. Baseline measures were determined then each participant was randomly assigned to either placebo or supplement followed by crossover to the other treatment.
Table 1
Participant descriptive characteristics
| Participants | N | Age (Mean ± SD) | Body mass (kg) | Body height (meters) |
|---|---|---|---|---|
| Female | 4 | 23.5 ± 9.1 | 57.6 ± 9.8 | 1.65 ± 0.09 |
| Male | 15 | 30.4 ± 6.6 | 82.5 ± 9.6 | 1.84 ± 0.08 |
SD: standard deviation.
2. Supplementation and blinding
Molecular hydrogen was administered via encapsulating H2-producing tablets (DrinkHRW), donated from HRW Natural Health Products Inc. (New Westminster BC, Canada), and then orally ingested with water. H2 is produced by the active ingredient metallic magnesium (80 mg), which reacts with water to produce H2 gas and magnesium hydroxide according to the reaction: Mg + 2H2O → H2 (g) + Mg(OH)2. The tablets also contain organic acids (i.e. malic acid and tartaric acid), which neutralize the magnesium hydroxide and catalyze the reaction rate. We determined the dose of molecular hydrogen administered by dissolving a tablet in a 500-mL beaker and immediately testing the hydrogen gas concentration with H2Blue (H2Sciences Inc. Las Vegas, NV) The concentration was determined to be super-saturated at ≈5 mM (10 mg/L), but would quickly decrease to its standard saturation of 0.8 mM (1.6 mg/L) within 30 min. Stoichiometrically, in a 500-mL container, each tablet with 80 mg of metallic magnesium, could provide a concentration of approximately 6.6 mM (13.1 mg/L) assuming all the magnesium reacts, and all the gas dissolves in the water. Therefore, each subject likely received around 2.5 milli-moles (5 mg) of H2.
The tablets react quickly with water to produce hydrogen gas, which is easily observed, therefore to ensure that participants and researchers were blinded to the treatments tablets were encapsulated and orally ingested by participants. Hydrogen and placebo capsules appeared identical. Placebo capsules, also donated by HRW Natural Health Products Inc. (New Westminster, BC, Canada), contained identical ingredients to the hydrogen supplement, but instead of metallic magnesium the placebo contained various forms of magnesium salts (i.e. tartrate, malate, chloride) and similar organic acids to prevent any pH buffering effect from the conjugate bases of the alkaline salts. The subjects ingested two capsules the night before and again at least 30 minutes before the testing, each capsule contained half a tablet.
3. Experimental protocol
A randomized, double-blinded, placebo-controlled cross-over design was used to evaluate the influence of acute supplementation of H2-producing tablets (DrinkHRW) compared to placebo, on maximal and submaximal indices of VO2, HR, RR, and RER. Each participant completed the testing protocol prior to being randomly assigned to ingest the H2-producing capsules (DrinkHRW) or placebo. Participants then repeated the protocol with the alternate treatment. There was a minimum of four days between each exercise test. Subjects were asked to maintain normal activity levels and dietary intake/timing for the duration of the study period. VO2 peak was determined via Bruce Protocol [30], wherein participants walked on the treadmill (10% grade) at an initial speed of 2.7 km/h followed by an increase in speed and grade every 3 minutes until volitional exhaustion. VO2 and RER were continuously measured with a metabolic measurement system (Ultima II, MedGraphics, St.Paul, MN). Each subject was equipped with a heart rate monitor (Polar CIC, Inc. NY), and HR was recorded every 30 seconds.
4. Statistical analysis
Dependent variables HR, RER, RR and VO2 were measured every 30 seconds for each “participant-time-from-start-of-exercise” combination under each condition (i.e. baseline, placebo and HRW). To determine effect of HRW on measures of exercise performance, a series of paired sample t-tests were used to compare HR, RR, RER and VO2 between conditions. Dependent variables (HR, RER, RR and VO2) were modeled as a function of the fixed effect of the condition (baseline, placebo and HRW) and the random effects of time and subject. Pairwise comparisons of baseline, placebo and HRW conditions were made and the Bonferroni adjustment was applied to the confidence intervals and significance values to account for multiple comparisons. The analysis was conducted for the entire sample, and for the subsamples limited to the range of 1–9 minutes, and from 9.5 minutes to the end of exercise. Summary statistics are shown in Table 2. All data were analyzed using 2-sided statistical tests (IBM SPSS Statistics 23). Statistical significance for the study was set a priori at alpha < 0.05.
Table 2
Summary statistics for the whole sample
| Variable | Total | 1–9 min | 9.5 min+ |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| HR-BL | 139.4 ± 33.2 | 124.3 ± 26.6 | 173.6 ± 17.7 |
| HR-PBO | 141.2 ± 32.3 | 125.9 ± 25.5 | 174.8 ± 16.2 |
| HR-HRW | 136.8 ± 33.3 | 120.8 ± 25.7 | 172.5 ± 15.6 |
| VO2-BL | 25.7 ± 10.6 | 20.4 ± 6.7 | 38.7 ± 6.3 |
| VO2-PBO | 24.2 ± 10.0 | 19.3 ± 6.6 | 36.3 ± 6.0 |
| VO2-HRW | 25.3 ± 10.4 | 20.1 ± 6.7 | 38.1 ± 5.6 |
| RER-BL | 1.0 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.2 |
| RER-PBO | 1.0 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.1 |
| RER-HRW | 1.0 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.1 |
| RR-BL | 29.5 ± 10.6 | 23.8 ± 5.8 | 41.5 ± 8.1 |
| RR-PBO | 29.6 ± 10.8 | 24.0 ± 5.4 | 41.4 ± 9.7 |
| RR-HRW | 29.1 ± 10.6 | 23.4 ± 5.9 | 41.0 ± 8.0 |
SE: standard deviation, HR: heart rate, BL: baseline, PBO: placebo, HRW: hydrogen-rich water, RER: respiratory exchange ratio, RR: respiratory rate.
RESULTS
The influence of molecular hydrogen on various exercise performance indices was evaluated during a graded treadmill exercise test to exhaustion. Data was collected for 19 subjects (15 males and 4 females) who underwent all three trials. The data of one subject was mistakenly not recorded and re-testing did not occur. Minimum and maximum values of HR, VO2, RER and RR were not significantly different across conditions (p > 0.05) (Table 3).
Table 3
Differences in minimum and maximum values of variables across three conditions
| Variable | Minimum | Max | ||
|---|---|---|---|---|
| F-statistic (df1 = 2, df2 = 36) | p-value | F-statistic (df1 = 2, df2 = 36) | p-value | |
| HR | 0.93 | 0.405 | 1.535 | 0.229 |
| VO2 | 0.496 | 0.612 | 1.110 | 0.341 |
| RER | 0.921 | 0.407 | 0.756 | 0.477 |
| RR | 0.940 | 0.400 | 1.802 | 0.180 |
HR: heart rate, RER: respiratory exchange ratio, RR: respiratory rate interval.
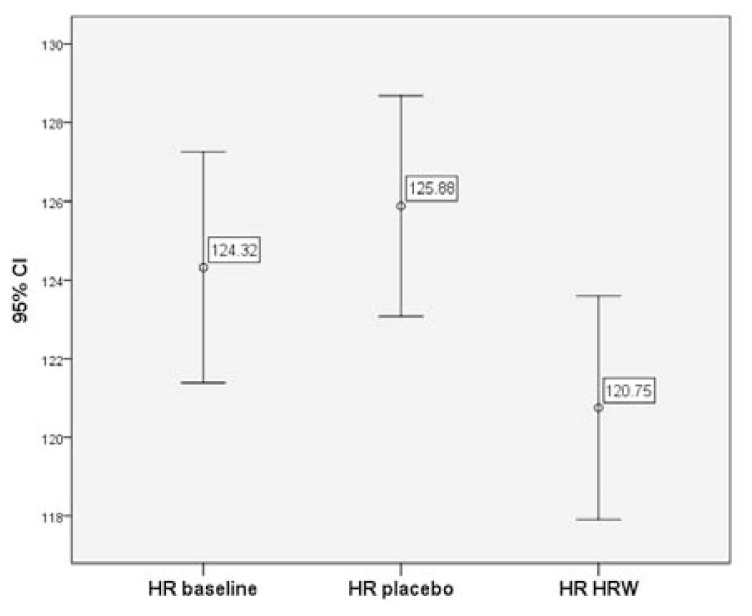
In contrast, analysis of mean HR from start of exercise (i.e. 1 min) to end of exercise revealed that there was a significant decrease in the average HR for HRW compared to both the baseline (≈3 bpm ± 0.4, p < 0.001) and the placebo (≈4 bpm ± 0.4, p < 0.001) (see Table 4). Placebo slightly increased HR (≈1 bpm ± 0.4) compared to baseline (p < 0.05). Compared to placebo, HRW had a slightly higher VO2 (p < 0.05), but was no different compared to baseline (p > 0.05). Placebo lowered VO2 compared to baseline (p < 0.05). However, the absolute value of the effect was small (≈1 mL *kg−1 * min−1 Pairwise differences in estimated marginal means of whole sample (1 min from start to end of exercise) with Bonferroni correction SE: standard error, CI: confidence interval, HRW: hydrogen-rich water, HR: heart rate, RER: respiratory exchange ratio, RR: respiratory rate interval. Pairwise differences in estimated marginal means with Bonferroni correction were also conducted at 1–9 minutes of exercise (see Table 5). On average, compared to baseline and placebo, HRW gave a lower HR (p < 0.001) by 3.6 bpm and 5.1 bpm (p < 0.001), respectively. HRW also significantly lowered the average RR compared to placebo (p = 0.018). However, compared to baseline, VO2 and RER were not changed by HRW (p > 0.05), while compared to placebo they were higher (p < 0.001) and lower (p < 0.001), respectively. However, in placebo, VO2 and RER were lower and higher, respectively compared to baseline (p < 0.001). The magnitude or effect size of these differences on all variables are rather small (Cohen’s d < 0.14). The mean HR and corresponding 95% confidence intervals for each group for the 1–9 minute subsample are presented in Fig. 1. Table 6 shows the similar calculated statistics with Bonferroni correction from 9.5 minutes until the end of exercise. Similar to measurements at 1–9 min and of the entire sample, HRW showed a slightly lower heart rate compared to baseline (p = 0.022) and placebo (p = 0.001). Compared to baseline, HRW did not influence VO2 (p > 0.05), but it was higher compared to placebo (p < 0.05), and placebo was lower than baseline (p < 0.05). RER, compared to baseline was slightly higher with HRW (p = 0.021), but not different compared to placebo (p > 0.05). RER was higher in placebo compared to baseline (p < 0.05). There was no statistically significant differences in RR in any of the groups. Pairwise differences in estimated marginal means of subsample: 1–9 minutes from the start with Bonferroni correction SE: standard error, CI: confidence interval, HRW: hydrogen-rich water, HR: heart rate, RER: respiratory exchange ratio, RR: respiratory rate interval. Pairwise differences in estimated marginal means of subsample: 9.5 minutes from the start to end of exercise with Bonferroni correction SE: standard error, CI: confidence interval, HRW: hydrogen-rich water, HR: heart rate, RER: respiratory exchange ratio, RR: respiratory rate interval.Table 4
Measure I J Mean difference (I–J) SE df p-value 95% CI Lower bound Upper bound HR HRW Baseline −2.93 0.43 1338.0 0.000 −3.96 −1.90 HR HRW Placebo −4.20 0.43 1337.9 0.000 −5.24 −3.17 HR Placebo Baseline 1.28 0.43 1338.0 0.009 0.24 2.31 VO2 HRW Baseline −0.35 0.18 1301.0 0.160 −0.79 0.08 VO2 HRW Placebo 1.09 0.18 1301.0 0.000 0.66 1.53 VO2 Placebo Baseline −1.45 0.18 1301.0 0.000 −1.88 −1.01 RER HRW Baseline −0.35 0.18 1301.0 0.160 −0.79 0.08 RER HRW Placebo 1.09 0.18 1301.0 0.000 0.66 1.53 RER Placebo Baseline −1.45 0.18 1301.0 0.000 −1.88 −1.01 RR HRW Baseline −0.46 0.23 1363.7 0.141 −1.02 0.09 RR HRW Placebo −0.63 0.23 1363.7 0.021 −1.18 −0.07 RR Placebo Baseline 0.17 0.23 1363.7 1.000 −0.39 0.72 Table 5
Measure I J Mean difference (I–J) SE df p-value 95% CI Lower bound Upper bound HR HRW Baseline −3.57 0.52 920.0 0.000 −4.81 −2.32 HR HRW Placebo −5.13 0.52 920.0 0.000 −6.37 −3.88 HR Placebo Baseline 1.56 0.52 920.0 0.008 0.32 2.81 VO2 HRW Baseline −0.26 0.17 919.9 0.403 −0.67 0.16 VO2 HRW Placebo 0.80 0.17 919.9 0.000 0.39 1.21 VO2 Placebo Baseline −1.06 0.17 919.9 0.000 −1.47 −0.64 RER HRW Baseline 0.01 0.00 920.0 1.000 −0.01 0.01 RER HRW Placebo −0.02 0.00 920.0 0.001 −0.03 −0.01 RER Placebo Baseline 0.02 0.00 920.0 0.000 0.01 0.03 RR HRW Baseline −0.42 0.24 920.0 0.256 −1.00 0.16 RR HRW Placebo −0.67 0.24 920.0 0.018 −1.25 −0.08 RR Placebo Baseline 0.25 0.24 920.0 0.919 −0.33 0.83 Table 6
Measure I J Mean Difference (I–J) SE df p-value 95% CI Lower bound Upper bound HR HRW Baseline −1.52 0.56 398.0 0.022 −2.87 −0.16 HR HRW Placebo −2.1 0.56 397.8 0.001 −3.44 −0.76 HR Placebo Baseline 0.58 0.56 398.0 0.910 −0.77 1.93 VO2 HRW Baseline −0.58 0.44 361.6 0.555 −1.63 0.47 VO2 HRW Placebo 1.82 0.44 361.8 0.000 0.77 2.86 VO2 Placebo Baseline −2.40 0.44 361.8 0.000 −3.44 −1.35 RER HRW Baseline 0.03 0.01 426.2 0.021 0.00 0.06 RER HRW Placebo −0.02 0.01 426.0 0.497 −0.04 0.01 RER Placebo Baseline 0.05 0.01 426.2 0.000 0.02 0.08 RR HRW Baseline −0.58 0.43 423.6 0.521 −1.61 0.44 RR HRW Placebo −0.58 0.43 423.6 0.536 −1.60 0.45 RR Placebo Baseline −0.01 0.43 423.6 1.000 −1.03 1.02
DISCUSSION
The effects of HRW on the exercise performance indices of HR, VO2, RER, and RR were evaluated during a maximal exercise treadmill test using a double-blinded, placebo-controlled cross-over study.
There were no significant differences in either HR, VO2, RR, or RER between trials at maximal or minimal exercise intensities. This suggests, HRW does not increase maximal HR, RR, VO2, or RER in young healthy adults. Several previous studies have shown that H2 improves submaximal exercise performance including reduction in fatigue and lactate levels [25,31]. We therefore analyzed the entire exercise trial and specific time intervals in order to determine the influence of HRW at various exercise intensities. Analysis of the entire sample revealed that HRW decreased the average exercising HR and RR and the effect on HR was especially pronounced at submaximal exercise intensities.
On average HRW decreased exercising HR by several beats per min while maintaining the same VO2 level compared to placebo. The Fick equation illustrates that VO2 = (stroke volume × HR) × (a-v) O2 difference. If HRW decreases exercise HR and VO2 is simultaneously maintained then we can speculate that HRW improves stroke volume and/or improves oxygen extraction and utilization at the muscle cell. It is unknown if H2 has such an effect. However, several exercise studies have shown that H2 decreases lactate levels [25,31]. Lactate production occurs when the ATP derived from mitochondrial oxidative phosphorylation cannot maintain the cellular energy demands. This requires oxidation of cytosolic NADH in order to produce ATP from glycolytic substrate-level phosphorylation. NAD+ is replenished by the reduction of pyruvate when forming lactate. Therefore, H2 may decrease lactate concentration by improving mitochondrial function, which would correspondingly increase oxygen extraction. However, maximal levels of VO2, HR, and RER were not significantly different, which suggests, in this population, HRW may only be effective at submaximal exercise intensities. More research is needed to confirm this observation and elucidate the molecular mechanisms.
Greater practical differences may have been evident if equal percentages of submaximal exercise had been compared. Additionally, instead of using time as the independent variable, using percentage of time, or percentage of maximal VO2 or HR as the independent variable may be more useful. For example, in our study, total exercise time was different for each participant due to varying levels of fitness and any specific minute mark corresponded to different percentages (e.g., 9 min corresponds to around 60% to 90%) of total exercise time depending on the participant. Instead of comparing the exercise indices at the same time interval future research should compare each value as a function of a specific exercise intensity (e.g., 70%) for each participant. This was not feasible in our study due to the problem of discreetness of measurement. Since these values were recorded every 30 seconds instead of more frequent sampling. Although prediction of the values by mathematical interpolation (y = mx + b) is possible, the differential between the 30 second intervals had substantial variability and linear interpolation would have been too inaccurate. Furthermore, the amount of time spent at a given intensity (e.g., 70%), was significantly different for each individual since the time to exhaustion during the graded-treadmill max test ranged from 10 to 15 minutes.
Future research should examine the effect of HRW on HR, VO2, RR, RER, and time to exhaustion at specific exercise intensities. Similarly, a range of distances could also be selected (e.g., 5 km or 10 km), and time trials could be performed with or without HRW. Future research should also determine the effect of HRW on rating of perceived exertion and blood lactate concentrations at various exercise intensities.
Our study was limited by a relatively small sample size and few female participants. The positive or the null effects of HRW may be different in older, younger, or diseased populations. Additionally, participants’ diet/calories and daily exercise patterns were not controlled or recorded nor was exact time of consumption of DrinkHRW or placebo capsules. Participants were asked to take the capsules the night before the test and at least approximately 30 min prior to the test. Perhaps the effects of HRW are altered if consumed in close conjunction with food since intestinal bacteria produce high levels of H2 upon metabolism of non-digestible carbohydrates [32]. The statistically significant benefits observed in this study and associated with HRW supplementation have unknown practical applications in regards to aerobic exercise performance and tolerance. The Cohen’s d of these changes suggest a small effect size; however, a better designed study may show greater practical significance to athletes. Additionally, small changes may become substantial under some scenarios (e.g., lower HR during marathon, elite athletic competitions, etc.).
CONCLUSION
Our results suggest that acute supplementation with molecular hydrogen does not improve maximal exercise performance in young healthy adults; however, it significantly reduces exercising HR at submaximal intensities. Although more studies are needed to determine the impact of HRW in diseased and non-diseased populations, the high safety profile of HRW coupled with this and previous studies, suggest that HRW could be a viable option as an ergogenic aid for athletes seeking to improve aerobic exercise performance.
ACKNOWLEDGEMENTS
We thank Mr. Alex Tarnava, CEO of HRW Natural Health Products Inc. for kindly donating DrinkHRW tablets for this study.
REFERENCES
Пълно съдържание на доклада:
original title: Acute Supplementation with Molecular Hydrogen Benefits Submaximal Exercise Indices. Randomized, Double-Blinded, Placebo-Controlled Crossover Pilot Study
Tyler W LaBaron, Abigail J Larson, Shigeo Ohta, Toshio Mikami, Jordon Barlow, Josh Bulloch, Mark DeBliso
-
Abstract:
Background: Clinical studies have reported hydrogen-rich water (HRW) to have therapeutic and ergogenic effects. The aim of this study was to determine the effect of acute supplementation with HRW on exercise performance as measured by VO2, respiratory exchange ratio (RER), heart rate (HR), and respiratory rate (RR).
Methods: Baseline levels of all exercise indices were determined in nineteen (4 female, 23.4 ± 9.1 yr; 15 male, 30.5 ± 6.8 yr) healthy subjects using a graded treadmill exercise test to exhaustion. Each subject was examined two additional times in a randomized double-blinded, placebo-controlled crossover fashion. Subjects received either HRW or placebo, which was consumed the day before and the day of the testing. HRW was delivered using the hydrogen-producing tablets, DrinkHRW (5 mg of H2). All data was analyzed with SPSS using pairwise comparisons with Bonferroni adjustment.
Results: HRW supplementation did not influence maximal or minimal indices of exercise performance (VO2, RER, HR and RR) (p < 0.05). However, HRW significantly decreased average exercising RR and HR (p < 0.05). HRW decreased exercising HR during minutes 1-9 of the graded exercise test (121 ± 26 bpm) compared to placebo (126 ± 26 bpm) and baseline (124 ± 27 bpm) (p < 0.001) without substantially influencing VO2.
Conclusion: Acute supplementation of DrinkHRW tablets may benefit submaximal aerobic exercise performance by lowering exercising HR. Further studies are needed to determine the influence and practical significance of HRW on varying exercise intensities as well as optimal dosing protocols and the effects of chronic use.